Biomass refers to organic materials derived from plants and animals, which can be used as a renewable energy source. It encompasses a wide range of organic matter, including wood, agricultural residues, crop waste, animal manure, and dedicated energy crops. Biomass is a versatile resource that can be converted into various forms of energy, including heat, electricity, and biofuels, through different conversion processes such as combustion, gasification, and anaerobic digestion.
Biomass Gasification
One of the key advantages of biomass is its abundance and widespread availability. It can be sustainably harvested and cultivated, making it a reliable and renewable source of energy. Biomass can also serve as a valuable alternative to fossil fuels, helping to reduce greenhouse gas emissions and mitigate climate change. By utilizing biomass for energy production, we can minimize our dependence on finite fossil fuel resources and transition towards a more sustainable and environmentally friendly energy system.
In addition to its energy potential, biomass offers several other benefits. For example, it can provide economic opportunities for rural communities by creating jobs in biomass production, harvesting, and processing. Biomass energy projects can also support local economies by providing revenue streams for farmers, foresters, and bioenergy producers.
However, it’s essential to consider the environmental and social implications of biomass utilization. While biomass can offer significant environmental benefits compared to fossil fuels, such as lower carbon emissions and reduced air pollution, it’s crucial to ensure that biomass is sourced and managed sustainably to avoid negative impacts on ecosystems, biodiversity, and food security. Sustainable biomass management practices, such as agroforestry, land-use planning, and waste-to-energy initiatives, can help maximize the benefits of biomass while minimizing its environmental footprint.
Overall, biomass represents a valuable renewable energy resource with the potential to play a significant role in our transition to a more sustainable energy future. By harnessing the power of biomass and investing in research, innovation, and infrastructure development, we can unlock its full potential as a clean, renewable, and environmentally friendly energy source.
Gasification:
Gasification is a thermochemical conversion process that converts carbonaceous materials, such as biomass, coal, or municipal solid waste, into synthesis gas (syngas) by reacting them with a controlled amount of oxygen, steam, or a combination of both at elevated temperatures. The process typically occurs in a gasifier, which is a reactor vessel designed to withstand high temperatures and pressures.
Gasification involves several chemical reactions, including pyrolysis, oxidation, and reduction, which occur in distinct zones within the gasifier. In the pyrolysis zone, the feedstock is heated in the absence of oxygen, leading to the release of volatile compounds such as hydrocarbons, tars, and methane. These volatile compounds are then partially oxidized in the oxidation zone, where they react with oxygen or steam to produce carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), and water vapor. Finally, in the reduction zone, carbon dioxide and water vapor react with carbonaceous char to produce additional syngas and carbon monoxide.
Syngas produced through gasification is a versatile fuel that can be used for a variety of applications, including electricity generation, heat production, and the synthesis of fuels and chemicals. It consists primarily of hydrogen and carbon monoxide, with trace amounts of methane, nitrogen, and other impurities. Syngas can be combusted directly in a gas turbine or reciprocating engine to generate electricity, or it can be further processed to remove impurities and separate hydrogen for use in fuel cells or chemical synthesis.
Gasification offers several advantages over other biomass conversion technologies. It can accommodate a wide range of feedstocks, including woody biomass, agricultural residues, and municipal solid waste, making it a flexible and versatile option for renewable energy production. Gasification also enables the efficient utilization of biomass resources by converting them into a high-energy-density syngas that can be easily transported and stored.
Furthermore, gasification is considered a more environmentally friendly option compared to traditional combustion processes, as it produces syngas with lower emissions of sulfur dioxide (SO2), nitrogen oxides (NOx), and particulate matter. The syngas can also be cleaned and conditioned to remove impurities such as tars, ammonia, and sulfur compounds, further reducing environmental impacts.
Despite its numerous advantages, gasification also presents technical and economic challenges. The process requires high temperatures and pressures, which can increase capital costs and energy consumption. Gasification reactors must also be carefully designed and operated to ensure efficient conversion and minimize the formation of tars and other byproducts. Additionally, the economics of gasification projects can be sensitive to factors such as feedstock availability, energy prices, and policy incentives.
In summary, gasification is a promising technology for converting biomass and other carbonaceous materials into clean and versatile syngas for renewable energy production. With ongoing research and development efforts focused on improving process efficiency, reducing costs, and addressing technical challenges, gasification has the potential to play a significant role in the transition to a more sustainable and low-carbon energy future.
Syngas:
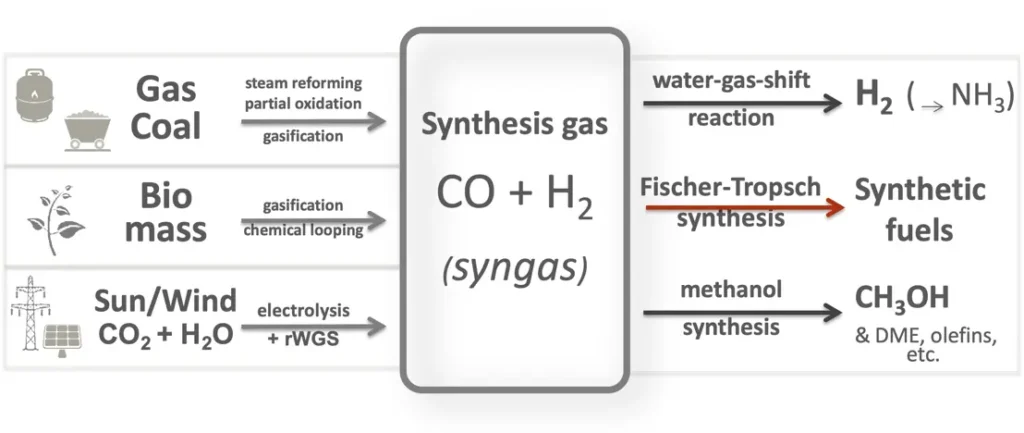
Syngas, short for synthesis gas, is a mixture of gases primarily composed of hydrogen (H2) and carbon monoxide (CO), along with varying amounts of carbon dioxide (CO2), methane (CH4), water vapor (H2O), and other trace gases. It is produced through thermochemical conversion processes such as gasification, pyrolysis, or steam reforming of carbonaceous feedstocks, including biomass, coal, natural gas, and municipal solid waste.
The composition of syngas depends on several factors, including the type of feedstock used, the operating conditions of the conversion process, and the presence of catalysts or additives. Syngas produced from biomass typically has a higher hydrogen-to-carbon monoxide ratio compared to syngas from coal or natural gas, making it suitable for a wider range of applications, including electricity generation, heat production, and the production of fuels and chemicals.
Syngas is a versatile and valuable intermediate product that can be used as a fuel or feedstock for various industrial processes. Some of the key applications of syngas include:
- Power Generation: Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity. Gasification-based power plants utilize syngas as a fuel to produce electricity with high efficiency and low emissions, making them an attractive option for decentralized and distributed power generation.
- Heat and Steam Production: Syngas can be used as a fuel for boilers, furnaces, and industrial heating applications to produce heat and steam for industrial processes, district heating systems, and cogeneration plants. Syngas-based heating systems offer an alternative to fossil fuels, reducing greenhouse gas emissions and reliance on imported energy sources.
- Fuels and Chemicals Production: Syngas serves as a feedstock for the production of fuels and chemicals through catalytic processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia synthesis. Syngas can be converted into liquid fuels such as synthetic diesel, gasoline, and jet fuel, as well as chemicals such as methanol, hydrogen, ammonia, and synthetic natural gas (SNG).
- Hydrogen Production: Syngas can be further processed through water-gas shift reactions or membrane separation to produce high-purity hydrogen for fuel cells, chemical synthesis, and industrial applications. Hydrogen derived from syngas is a clean and versatile energy carrier with potential applications in transportation, energy storage, and renewable energy integration.
Syngas production and utilization offer several advantages, including resource diversification, energy security, and environmental sustainability. By utilizing syngas as a renewable energy source, we can reduce our dependence on fossil fuels, mitigate climate change, and promote a more sustainable and low-carbon energy future. However, it’s essential to address technical, economic, and environmental challenges associated with syngas production and utilization, such as gasification efficiency, carbon capture and storage, and life cycle emissions, to maximize the potential benefits of this versatile energy resource.
Feedstock:
Feedstock refers to the raw material or biomass source used as input in various industrial processes, particularly in the context of renewable energy production, biofuel manufacturing, and chemical synthesis. It encompasses a wide range of organic materials derived from plants, animals, and organic wastes, which can be converted into energy, fuels, chemicals, and other valuable products through different conversion technologies and processes.
In the context of renewable energy and bioenergy production, feedstock plays a crucial role in determining the availability, cost, and sustainability of the resulting energy products. Different types of feedstocks have varying compositions, energy contents, and conversion potentials, influencing the choice of conversion technology, process efficiency, and environmental impacts of the energy production process.
Common types of feedstocks used in renewable energy and bioenergy production include:
- Biomass Feedstocks: Biomass feedstocks consist of organic materials derived from plants, trees, crops, agricultural residues, forestry residues, and dedicated energy crops. Biomass feedstocks can be used in various conversion processes, such as combustion, gasification, pyrolysis, and anaerobic digestion, to produce heat, electricity, biofuels, and bioproducts.
- Woody Biomass: Woody biomass includes wood chips, sawdust, logging residues, and tree trimmings obtained from forestry operations, timber processing, and urban wood waste. Woody biomass feedstocks are widely used in biomass-fired power plants, district heating systems, and biomass boilers for heat and electricity generation.
- Agricultural Residues: Agricultural residues are byproducts of agricultural crops and farming activities, such as crop residues, straw, husks, stalks, and bagasse. Agricultural residues can be used as feedstocks for bioenergy production through processes like biomass combustion, gasification, and bioethanol fermentation.
- Energy Crops: Energy crops are dedicated biomass crops grown specifically for bioenergy production, such as switchgrass, miscanthus, willow, and poplar. Energy crops offer high yields of biomass per hectare and can be cultivated on marginal lands unsuitable for food crops, providing a sustainable and renewable source of feedstock for bioenergy production.
- Animal Manure and Wastes: Animal manure, livestock waste, and organic residues from agricultural and livestock operations can be used as feedstocks for anaerobic digestion to produce biogas and renewable natural gas (RNG). Anaerobic digestion of organic waste materials not only generates renewable energy but also helps reduce methane emissions from waste decomposition and provides nutrient-rich digestate for soil amendment.
- Municipal Solid Waste (MSW): Municipal solid waste, including household waste, commercial waste, and industrial waste, can be used as a feedstock for waste-to-energy (WtE) facilities, such as waste incineration plants and landfill gas recovery systems. MSW feedstocks can be converted into electricity, heat, and biofuels through combustion, gasification, or landfill gas capture.
The choice of feedstock for renewable energy production depends on factors such as feedstock availability, cost, quality, sustainability, and local regulations. Sustainable feedstock sourcing practices, including responsible land management, crop rotation, and waste diversion, are essential to ensure the long-term viability and environmental sustainability of bioenergy feedstock supply chains. Additionally, advancements in feedstock pretreatment, conversion technologies, and process optimization can improve the efficiency, economics, and environmental performance of renewable energy production from diverse feedstock sources.
Thermochemical:
Thermochemical processes involve the transformation of chemical substances through the application of heat. In the context of renewable energy and bioenergy production, thermochemical processes play a crucial role in converting biomass and other organic materials into useful energy carriers such as syngas, biofuels, and heat. These processes utilize heat to drive chemical reactions that break down complex organic molecules into simpler compounds, releasing energy in the form of heat, gases, and liquids.
Key thermochemical processes used in renewable energy and bioenergy production include:
- Combustion: Combustion is a thermochemical process that involves the rapid oxidation of biomass or fossil fuels in the presence of oxygen to release heat energy. In biomass combustion, organic materials such as wood, agricultural residues, and bioenergy crops are burned in a controlled environment, typically in boilers, furnaces, or stoves, to produce heat for space heating, water heating, and electricity generation.
- Gasification: Gasification is a thermochemical conversion process that converts carbonaceous feedstocks, such as biomass, coal, or waste materials, into a mixture of gases known as syngas. The process occurs in a gasifier reactor, where the feedstock is subjected to high temperatures and a controlled amount of oxygen or steam, leading to the partial oxidation of organic matter and the production of syngas.
- Pyrolysis: Pyrolysis is a thermochemical decomposition process that breaks down organic materials into biochar, bio-oil, and syngas in the absence of oxygen or with limited oxygen supply. During pyrolysis, biomass feedstocks are heated to high temperatures in an oxygen-starved environment, causing them to undergo thermal degradation and chemical decomposition, resulting in the formation of biochar, a solid carbon-rich residue, bio-oil, a liquid mixture of organic compounds, and syngas.
- Steam Reforming: Steam reforming, also known as steam methane reforming (SMR), is a thermochemical process used to produce hydrogen gas (H2) from natural gas, biogas, or other hydrocarbon feedstocks. The process involves reacting methane (CH4) with steam (H2O) at high temperatures and pressures in the presence of a catalyst to produce hydrogen gas and carbon monoxide.
- Biomass Liquefaction: Biomass liquefaction is a thermochemical process that converts biomass into liquid fuels such as bio-oil, biodiesel, and synthetic fuels through the application of heat and pressure. The process involves heating biomass feedstocks in the presence of a solvent or catalyst to break down complex organic molecules into liquid hydrocarbons, which can be upgraded and refined into transportation fuels.
Thermochemical processes offer several advantages for renewable energy and bioenergy production, including high energy efficiency, versatility in feedstock utilization, and the ability to produce a range of energy carriers and products. These processes can also help reduce greenhouse gas emissions, promote resource efficiency, and enhance energy security by utilizing renewable and sustainable feedstocks.
However, thermochemical processes also present challenges such as high capital costs, complex process integration, and environmental concerns related to emissions, waste management, and byproduct utilization. Addressing these challenges requires ongoing research and development efforts focused on improving process efficiency, reducing costs, and optimizing environmental performance to realize the full potential of thermochemical technologies for renewable energy and bioenergy production.
Biomass-to-energy:
Biomass-to-energy refers to the process of converting biomass resources into usable energy forms such as heat, electricity, or biofuels. It involves a variety of conversion technologies and processes that harness the energy stored in organic materials derived from plants, animals, and organic wastes. Biomass-to-energy technologies play a crucial role in the transition to a more sustainable and renewable energy system by utilizing locally available biomass resources to produce clean and renewable energy while reducing greenhouse gas emissions and dependence on fossil fuels.
There are several biomass-to-energy conversion technologies, each suited to different types of biomass feedstocks and energy applications:
- Combustion: Biomass combustion is one of the oldest and most widely used biomass-to-energy technologies. It involves burning biomass feedstocks such as wood, agricultural residues, and energy crops in a controlled environment to produce heat. The heat generated can be used directly for space heating, water heating, or industrial processes, or it can be converted into electricity using steam turbines or other power generation technologies.
- Gasification: Biomass gasification is a thermochemical conversion process that converts biomass feedstocks into a combustible gas mixture known as syngas. The process involves heating biomass in a gasifier reactor with a controlled amount of oxygen or steam, resulting in the partial oxidation of organic matter and the production of syngas. Syngas can be used as a fuel for power generation, combined heat and power (CHP) systems, or as a feedstock for the production of biofuels and chemicals.
- Pyrolysis: Biomass pyrolysis is a thermochemical decomposition process that converts biomass feedstocks into biochar, bio-oil, and syngas in the absence of oxygen or with limited oxygen supply. During pyrolysis, biomass is heated to high temperatures in a low-oxygen environment, causing it to undergo thermal degradation and chemical decomposition. The resulting products, including biochar for soil amendment, bio-oil for biofuel production, and syngas for energy generation, have various applications in energy, agriculture, and industry.
- Anaerobic Digestion: Biomass anaerobic digestion is a biological conversion process that breaks down organic materials in the absence of oxygen to produce biogas, a mixture of methane and carbon dioxide. The process occurs in anaerobic digesters, where microorganisms decompose organic matter, such as animal manure, agricultural residues, and food waste, to produce biogas. Biogas can be used as a renewable fuel for electricity generation, heating, or vehicle fuel, or it can be upgraded to biomethane for injection into natural gas pipelines or use as a transportation fuel.
- Biochemical Conversion: Biomass biochemical conversion involves using enzymes or microorganisms to convert biomass feedstocks into biofuels such as ethanol, biodiesel, and biobutanol through fermentation, enzymatic hydrolysis, or other biochemical processes. These biofuels can be used as renewable alternatives to gasoline, diesel, and other fossil fuels in transportation, industry, and agriculture.
Biomass-to-energy technologies offer several advantages, including carbon neutrality, renewable energy generation, waste reduction, and rural development opportunities. By utilizing locally available biomass resources, biomass-to-energy projects can enhance energy security, promote economic growth, and support sustainable development goals. However, challenges such as feedstock availability, technology costs, and environmental impacts must be addressed to realize the full potential of biomass-to-energy as a clean and sustainable energy solution. Ongoing research, innovation, and policy support are essential to overcome these challenges and advance the deployment of biomass-to-energy technologies worldwide.
Renewable Fuel:
Renewable fuel refers to any fuel derived from renewable or sustainable sources, such as biomass, solar energy, wind energy, or hydropower. Unlike fossil fuels, which are finite and contribute to environmental degradation and climate change, renewable fuels are produced from resources that can be replenished naturally and do not deplete finite resources or contribute to greenhouse gas emissions when used.
Key types of renewable fuels include biofuels, hydrogen, and renewable electricity:
- Biofuels: Biofuels are liquid or gaseous fuels derived from biomass feedstocks such as crops, agricultural residues, forestry residues, algae, and organic wastes. Common biofuels include ethanol, biodiesel, biogas, and renewable diesel. Ethanol is produced through fermentation of sugars or starches found in crops such as corn, sugarcane, and wheat, while biodiesel is produced from vegetable oils or animal fats through a process called transesterification. Biogas is generated through anaerobic digestion of organic wastes such as animal manure, food scraps, and wastewater sludge.
- Hydrogen: Hydrogen is a versatile renewable fuel that can be produced from water through electrolysis, biomass gasification, or thermochemical processes. It can be used as a fuel for fuel cells to generate electricity or as a feedstock for chemical processes to produce synthetic fuels such as hydrogenated vegetable oil (HVO) or ammonia. Hydrogen fuel cells offer high efficiency, zero emissions, and quiet operation, making them suitable for various applications, including transportation, stationary power generation, and energy storage.
- Renewable Electricity: Renewable electricity is generated from renewable energy sources such as solar, wind, hydro, geothermal, and biomass. It is produced through technologies such as photovoltaic (PV) solar panels, wind turbines, hydroelectric dams, and biomass power plants. Renewable electricity can be used directly to power electric vehicles, heat pumps, and appliances, or it can be stored in batteries or converted into other forms of energy such as hydrogen through electrolysis. Renewable electricity is a clean and sustainable alternative to fossil fuel-based electricity generation, offering environmental benefits such as reduced greenhouse gas emissions, air pollution, and water usage.
Renewable fuels offer several advantages over conventional fossil fuels, including:
- Environmental Benefits: Renewable fuels are derived from sustainable sources and produce lower emissions of greenhouse gases, air pollutants, and toxic substances compared to fossil fuels. Using renewable fuels can help mitigate climate change, improve air quality, and reduce environmental impacts associated with fossil fuel extraction, processing, and combustion.
- Energy Security: Renewable fuels reduce dependence on imported fossil fuels and enhance energy security by utilizing locally available resources and diversifying energy sources. Renewable fuels can be produced domestically, reducing reliance on foreign oil and enhancing energy independence and resilience.
- Economic Opportunities: Renewable fuel production and deployment create economic opportunities for farmers, rural communities, and clean energy industries. Renewable fuel projects support job creation, economic growth, and investment in renewable energy infrastructure, contributing to local economies and sustainable development.
- Technological Innovation: Renewable fuels drive technological innovation and advancements in renewable energy technologies, including bioenergy, hydrogen, and renewable electricity generation. Research and development efforts focused on improving renewable fuel production processes, efficiency, and cost-effectiveness help drive down costs and accelerate the transition to a clean energy future.
Overall, renewable fuels play a crucial role in transitioning to a more sustainable, low-carbon energy system by offering clean, renewable alternatives to conventional fossil fuels. By promoting the development and deployment of renewable fuels, policymakers, businesses, and consumers can contribute to reducing greenhouse gas emissions, promoting energy security, and fostering economic growth while ensuring a sustainable future for generations to come.
Tar:
Tar, in the context of biomass gasification and pyrolysis processes, refers to a complex mixture of organic compounds that are produced as byproducts during the thermal conversion of biomass feedstocks. It is a viscous, sticky substance with high molecular weight and can vary widely in composition depending on the feedstock, operating conditions, and process parameters.
During biomass gasification or pyrolysis, organic materials undergo thermal decomposition in the absence of oxygen or with limited oxygen supply, leading to the release of volatile compounds such as gases (e.g., carbon monoxide, hydrogen, methane) and condensable vapors. These condensable vapors, also known as tar, consist of a wide range of organic compounds, including polycyclic aromatic hydrocarbons (PAHs), phenols, aldehydes, ketones, and organic acids.
Tar formation occurs through a series of complex chemical reactions involving the pyrolysis and decomposition of biomass constituents such as cellulose, hemicellulose, lignin, and other organic compounds present in the feedstock. Factors influencing tar formation include temperature, residence time, heating rate, biomass composition, moisture content, reactor design, and gasification/pyrolysis conditions.
Tar poses several challenges in biomass gasification and pyrolysis processes, including:
- Equipment Fouling: Tar can condense on reactor walls, heat exchangers, and downstream equipment, leading to fouling and corrosion. Accumulation of tar deposits reduces heat transfer efficiency, decreases process performance, and increases maintenance requirements, resulting in downtime and higher operating costs.
- Catalyst Deactivation: Tar can deactivate catalysts used in downstream processing units or gas cleaning systems, reducing their effectiveness and catalytic activity. Tar deposition on catalyst surfaces inhibits active sites and pore blockage, leading to decreased catalyst performance and shorter catalyst lifetimes.
- Gas Quality and Composition: Tar contaminants in syngas can adversely affect downstream gas utilization equipment such as engines, turbines, and fuel cells. High tar levels in syngas can cause engine fouling, corrosion, and tar condensation in combustion chambers, leading to engine damage, reduced efficiency, and increased emissions.
- Environmental and Health Concerns: Tar contains toxic and carcinogenic compounds such as PAHs, benzene, toluene, and xylene, posing environmental and health risks to workers, communities, and ecosystems. Tar emissions from biomass gasification and pyrolysis processes contribute to air pollution, smog formation, and respiratory illnesses, necessitating emission control measures and environmental regulations.
To mitigate the adverse effects of tar, various tar removal and mitigation strategies are employed, including:
- Tar Cracking: Tar cracking involves catalytic or non-catalytic decomposition of tar molecules into lighter gases such as methane, hydrogen, and carbon monoxide at high temperatures. Tar cracking reactors or reformers are integrated into gasification or pyrolysis systems to reduce tar levels and improve syngas quality.
- Tar Removal and Conditioning: Tar removal techniques such as filtration, scrubbing, condensation, and catalytic tar reforming are used to remove tar from syngas streams and convert it into useful products or inert solids. These tar removal systems are integrated into gasification or pyrolysis plants to improve gas quality and protect downstream equipment.
- Biomass Pretreatment: Biomass pretreatment techniques such as drying, torrefaction, and pelletization can reduce tar formation by removing moisture, volatiles, and reactive components from the feedstock. Pretreated biomass exhibits improved handling properties, thermal stability, and gasification/pyrolysis performance, resulting in lower tar yields and enhanced process efficiency.
Addressing tar formation and management is essential to the successful operation of biomass gasification and pyrolysis processes for renewable energy production. Research and development efforts focused on tar characterization, detection, mitigation, and utilization continue to advance tar management strategies and improve the efficiency, reliability, and environmental performance of biomass conversion technologies.
Syngas Cleanup:
Syngas cleanup refers to the process of removing impurities and contaminants from synthesis gas (syngas) produced during biomass gasification, pyrolysis, or other thermochemical conversion processes. Syngas cleanup is essential to ensure the quality, purity, and stability of syngas before it can be utilized as a fuel or feedstock for downstream applications such as power generation, heat production, or chemical synthesis.
Syngas produced from biomass gasification or pyrolysis typically contains various impurities and contaminants, including tar, particulates, sulfur compounds, ammonia, alkali metals, trace metals, and moisture. These impurities can adversely affect the performance, efficiency, and reliability of syngas utilization equipment such as engines, turbines, fuel cells, and catalysts, leading to equipment fouling, corrosion, catalyst deactivation, and increased maintenance requirements.
Syngas cleanup processes typically involve a combination of physical, chemical, and thermal treatment steps to remove or reduce impurities to acceptable levels. Key syngas cleanup technologies and techniques include:
- Particulate Removal: Particulate matter such as ash, dust, and char particles are removed from syngas using filtration, cyclone separators, or electrostatic precipitators. Particulate removal helps protect downstream equipment from fouling and erosion and improves syngas quality and cleanliness.
- Tar Removal: Tar compounds present in syngas are removed through tar cracking, tar reforming, or tar removal systems such as filters, scrubbers, or condensers. Tar removal prevents fouling and corrosion of downstream equipment and enhances syngas stability and combustion performance.
- Sulfur Removal: Sulfur compounds such as hydrogen sulfide (H2S) and carbonyl sulfide (COS) are removed from syngas using desulfurization techniques such as absorption, adsorption, or catalytic conversion. Sulfur removal prevents corrosion, catalyst poisoning, and environmental emissions and enables syngas utilization in sensitive applications such as fuel cells and chemical synthesis.
- Ammonia Removal: Ammonia (NH3) present in syngas is removed through selective catalytic reduction (SCR) or scrubbing with acidic solutions. Ammonia removal prevents catalyst poisoning, corrosion, and environmental emissions and improves syngas quality for downstream applications.
- Moisture Removal: Moisture content in syngas is reduced using condensation, cooling, or dehydration techniques such as membrane separation or molecular sieves. Moisture removal prevents corrosion, ice formation, and gasifier fouling and improves syngas storage and handling properties.
- Gas Composition Adjustment: Syngas composition is adjusted by controlling the ratio of hydrogen (H2) to carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other gases through syngas conditioning, reforming, or catalytic conversion. Gas composition adjustment ensures syngas meets specific process requirements and enables efficient utilization in end-use applications.
Syngas cleanup is an essential and often complex step in biomass-to-energy conversion processes, requiring careful selection and integration of cleanup technologies to achieve desired syngas quality, purity, and stability. Advances in syngas cleanup technologies, process optimization, and control systems continue to improve the efficiency, reliability, and environmental performance of biomass gasification and pyrolysis plants, enabling the widespread adoption of renewable syngas as a clean and sustainable energy source.
Syngas Utilization:
Syngas utilization refers to the conversion and utilization of synthesis gas (syngas) produced from biomass gasification, pyrolysis, or other thermochemical processes as a valuable energy carrier or feedstock for various industrial applications. Syngas is a versatile intermediate product containing hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other trace gases, making it suitable for a wide range of energy and chemical production processes.
Key syngas utilization pathways include:
- Power Generation: Syngas can be utilized as a fuel for power generation through combustion in gas turbines, reciprocating engines, or fuel cells. Gasification-based power plants use syngas to generate electricity efficiently with low emissions, offering a renewable and flexible energy solution for decentralized and distributed power generation applications.
- Combined Heat and Power (CHP): Syngas can be used for combined heat and power (CHP) applications, where it is simultaneously used for electricity generation and heat production. CHP systems utilize the waste heat generated during electricity generation for space heating, water heating, or industrial processes, improving overall energy efficiency and reducing energy costs.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, biodiesel, and synthetic diesel through catalytic processes such as Fischer-Tropsch synthesis, methanol synthesis, and alcohol synthesis. Biofuels produced from syngas offer renewable alternatives to fossil fuels for transportation, industry, and agriculture, reducing greenhouse gas emissions and dependence on imported oil.
- Chemical Synthesis: Syngas is a key feedstock for the production of various chemicals and industrial products through catalytic processes such as ammonia synthesis, methanol synthesis, and hydrocarbon synthesis. Chemicals derived from syngas include ammonia for fertilizer production, methanol for chemical synthesis and fuel blending, and synthetic hydrocarbons for plastics, polymers, and specialty chemicals.
- Hydrogen Production: Syngas can be further processed through water-gas shift reactions or membrane separation to produce high-purity hydrogen gas (H2) for fuel cells, chemical synthesis, and industrial applications. Hydrogen derived from syngas serves as a clean and versatile energy carrier with applications in transportation, energy storage, and renewable energy integration.
- Synthetic Natural Gas (SNG): Syngas can be upgraded and converted into synthetic natural gas (SNG) through methanation processes, where carbon monoxide (CO) and hydrogen (H2) are reacted to produce methane (CH4). SNG can be injected into natural gas pipelines or used as a renewable substitute for natural gas in heating, cooking, and industrial processes.
Syngas utilization offers several advantages, including resource diversification, energy security, and environmental sustainability. By utilizing syngas as a renewable energy source and feedstock for chemical synthesis, we can reduce our dependence on fossil fuels, mitigate climate change, and promote a more sustainable and low-carbon energy future. However, challenges such as syngas quality, process efficiency, and market integration must be addressed to maximize the economic and environmental benefits of syngas utilization across various sectors. Ongoing research, innovation, and policy support are essential to accelerate the adoption and deployment of syngas utilization technologies worldwide.
Biomass Gasification:
Biomass gasification is a thermochemical conversion process that transforms biomass feedstocks into a gaseous mixture known as syngas (synthesis gas), which consists primarily of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and trace amounts of other gases. This process occurs in the absence of oxygen or with limited oxygen supply, distinguishing it from traditional combustion processes.
The biomass gasification process typically involves several key steps:
- Feedstock Preparation: Biomass feedstocks, which can include wood chips, agricultural residues, energy crops, and municipal solid waste, are prepared for gasification by reducing particle size and moisture content. This preparation step enhances the efficiency of biomass conversion and facilitates uniform feeding into the gasifier.
- Gasification Reactor: The prepared biomass feedstock is introduced into a gasifier reactor, where it undergoes thermal decomposition and chemical reactions at high temperatures (typically between 700°C and 1,200°C) in the presence of a controlled amount of steam, oxygen, or a combination of both. The primary reactions that occur during biomass gasification include pyrolysis, partial oxidation, and reduction:
- Pyrolysis: Biomass feedstocks are heated in the absence of oxygen, leading to the release of volatile organic compounds and the formation of char.
- Partial Oxidation: Volatile compounds released during pyrolysis react with oxygen or steam to produce carbon monoxide (CO) and hydrogen (H2) through endothermic reactions.
- Reduction: Carbon monoxide (CO) reacts with steam (H2O) or carbon dioxide (CO2) to produce additional hydrogen (H2) and carbon dioxide (CO2), along with smaller amounts of methane (CH4) and other trace gases.
- Syngas Cleanup: The raw syngas produced in the gasifier contains impurities such as tar, particulates, sulfur compounds, and ammonia, which must be removed or reduced to acceptable levels to prevent equipment fouling, corrosion, and environmental emissions. Syngas cleanup technologies include tar cracking, particulate removal, desulfurization, ammonia scrubbing, and moisture removal.
- Syngas Conditioning: The cleaned syngas may undergo further conditioning and treatment to adjust its composition, temperature, and pressure to meet specific process requirements and optimize its utilization in downstream applications. Syngas conditioning may involve cooling, compression, moisture removal, and gas composition adjustment through catalytic conversion or chemical reactions.
- Syngas Utilization: The conditioned syngas is utilized as a renewable energy source or feedstock for various industrial applications, including power generation, combined heat and power (CHP) systems, biofuels production, chemical synthesis, hydrogen production, and synthetic natural gas (SNG) production. Syngas utilization pathways depend on factors such as syngas composition, quality, and end-use requirements.
Biomass gasification offers several advantages over traditional combustion and other biomass conversion processes, including higher energy efficiency, lower emissions, greater fuel flexibility, and the ability to produce a versatile energy carrier (syngas) for multiple applications. Gasification technology has the potential to play a significant role in the transition to a more sustainable and low-carbon energy system by utilizing abundant biomass resources to produce clean and renewable energy while reducing greenhouse gas emissions and dependence on fossil fuels. Ongoing research, development, and deployment efforts are essential to advance biomass gasification technology, improve process efficiency, reduce costs, and expand its commercial viability for widespread adoption in various sectors.
Gasification Plant
A gasification plant is an industrial facility designed to convert various carbonaceous feedstocks, such as biomass, coal, petroleum coke, or waste materials, into synthesis gas (syngas) through the process of gasification. Gasification plants play a crucial role in the production of clean and renewable energy, as well as in the synthesis of chemicals, fuels, and other valuable products.
Key components and processes involved in a gasification plant include:
- Feedstock Handling and Preparation: The gasification process begins with the handling and preparation of the feedstock. Depending on the type of feedstock used (e.g., wood chips, coal, municipal solid waste), feedstock preparation may involve size reduction (shredding or grinding), drying to reduce moisture content, and removal of contaminants or impurities.
- Gasifier Reactor: The prepared feedstock is fed into a gasifier reactor, where it undergoes thermal decomposition and chemical reactions in a controlled environment. Gasifier designs vary, but most operate at high temperatures (typically between 700°C and 1,200°C) and pressures, and use a controlled amount of oxygen, steam, or a combination of both to convert the feedstock into syngas.
- Syngas Cleanup: The raw syngas produced in the gasifier contains impurities such as tar, particulates, sulfur compounds, ammonia, and moisture, which must be removed or reduced to acceptable levels. Syngas cleanup processes include tar cracking, particulate removal (e.g., cyclones, filters), desulfurization, ammonia scrubbing, and moisture removal (e.g., condensation, absorption).
- Syngas Conditioning: After cleanup, the syngas may undergo further conditioning and treatment to adjust its temperature, pressure, and composition to meet specific process requirements and optimize its utilization in downstream applications. Syngas conditioning processes may include cooling, compression, moisture removal, and gas composition adjustment through catalytic conversion or chemical reactions.
- Syngas Utilization: The conditioned syngas is utilized as a renewable energy source or feedstock for various industrial applications. Common syngas utilization pathways include:
- Power Generation: Syngas is combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity.
- Combined Heat and Power (CHP): Syngas is used for combined heat and power applications, where waste heat from electricity generation is utilized for heating or industrial processes.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, biodiesel, and synthetic diesel through catalytic processes.
- Chemical Synthesis: Syngas is used as a feedstock for the production of chemicals and industrial products through catalytic processes such as ammonia synthesis, methanol synthesis, and Fischer-Tropsch synthesis.
Gasification plants offer several advantages over conventional combustion-based power plants, including higher energy efficiency, lower emissions, greater fuel flexibility, and the ability to utilize a wide range of feedstocks, including biomass and waste materials. Gasification technology has the potential to play a significant role in the transition to a more sustainable and low-carbon energy system by enabling the production of clean and renewable energy from abundant and diverse carbonaceous resources. Ongoing research, development, and deployment efforts are essential to advance gasification technology, improve process efficiency, reduce costs, and enhance its commercial viability for widespread adoption in various sectors.
Biomass Feedstock:
Biomass feedstock refers to organic materials derived from plants, animals, or organic wastes that are used as raw materials for biomass conversion processes, such as gasification, pyrolysis, fermentation, or combustion, to produce energy, fuels, chemicals, or other valuable products. Biomass feedstocks are renewable resources that can be sustainably harvested, cultivated, or obtained from agricultural, forestry, industrial, and municipal sources.
There are various types of biomass feedstocks, each with unique characteristics, availability, and suitability for different conversion processes:
- Woody Biomass: Woody biomass includes trees, shrubs, branches, bark, and wood residues obtained from forestry operations, sawmills, lumber yards, and urban tree trimmings. Woody biomass feedstocks, such as wood chips, sawdust, wood pellets, and logging residues, are commonly used in biomass gasification, combustion, and pelletization processes to produce heat, electricity, or biofuels.
- Agricultural Residues: Agricultural residues are byproducts of agricultural production processes, such as crop residues, straw, husks, stalks, bagasse, and hulls. These residues are obtained from crops such as corn, wheat, rice, sugarcane, and soybeans and can be used as feedstocks for biomass gasification, pyrolysis, or combustion to generate heat, power, or biofuels.
- Energy Crops: Energy crops are dedicated biomass crops grown specifically for energy production purposes. Examples of energy crops include switchgrass, miscanthus, willow, poplar, and hybrid poplar. Energy crops can be cultivated on marginal lands or fallow agricultural fields and harvested for use in biomass conversion processes to produce biofuels or bioproducts.
- Municipal Solid Waste (MSW): Municipal solid waste, also known as garbage or trash, consists of household, commercial, and industrial wastes collected from urban and municipal areas. MSW feedstocks include organic materials such as food waste, yard waste, paper, cardboard, and other biodegradable materials. MSW can be processed through anaerobic digestion, composting, or waste-to-energy technologies to produce biogas, compost, or electricity.
- Industrial Wastes: Industrial wastes are byproducts generated from industrial processes, such as manufacturing, food processing, pulp and paper production, and wastewater treatment. Industrial waste feedstocks include organic residues, sludges, byproducts, and waste streams rich in organic matter. These wastes can be utilized as feedstocks for biomass conversion processes to recover energy or produce value-added products.
- Animal Manure: Animal manure is organic waste produced by livestock, poultry, and dairy operations. Manure contains organic matter, nutrients, and pathogens and can be processed through anaerobic digestion or composting to produce biogas, biofertilizers, or soil amendments. Anaerobic digestion of animal manure generates biogas, a renewable fuel consisting primarily of methane and carbon dioxide.
Biomass feedstocks offer several advantages as renewable and sustainable resources for energy production and waste management, including carbon neutrality, resource diversification, rural development, and waste reduction. However, challenges such as feedstock availability, logistics, handling, and sustainability must be addressed to ensure the efficient and environmentally responsible utilization of biomass feedstocks in biomass conversion processes. Ongoing research, technology development, and policy support are essential to promote the sustainable use of biomass feedstocks and advance the deployment of biomass-based energy systems worldwide.
Fluidized Bed Gasifier:
A fluidized bed gasifier is a type of gasification reactor that utilizes a fluidized bed technology to convert solid biomass feedstocks into synthesis gas (syngas) by subjecting them to high temperatures and controlled air or oxygen flow in a fluidized state. This technology is widely used in biomass-to-energy conversion systems for the production of heat, power, biofuels, and chemicals.
Key features and components of a fluidized bed gasifier include:
- Fluidized Bed Reactor: The heart of the fluidized bed gasifier is the reactor vessel, which contains a bed of inert particles (e.g., sand, alumina, dolomite) that are fluidized by a stream of gas (usually air or oxygen) entering from the bottom of the reactor. The fluidization process suspends the solid biomass feedstock particles within the bed, creating a fluidized bed of solids with properties similar to a boiling liquid.
- Biomass Feeding System: Solid biomass feedstocks, such as wood chips, agricultural residues, or energy crops, are introduced into the fluidized bed reactor through a feeding system, which may include screw conveyors, rotary valves, or pneumatic conveyors. The biomass feedstock is continuously fed into the reactor to maintain a steady gasification process.
- Gasification Zone: The biomass feedstock undergoes thermal decomposition, pyrolysis, and chemical reactions as it moves through the fluidized bed reactor. Heat is supplied to the reactor through direct combustion of a portion of the biomass or by injecting hot gases into the bed. The high temperature (typically between 700°C and 1,200°C) promotes the gasification reactions, resulting in the production of syngas.
- Gasification Reactions: In the fluidized bed gasifier, biomass feedstock particles are converted into syngas through a series of thermochemical reactions, including:
- Pyrolysis: Biomass feedstock is heated in the absence of oxygen, leading to the release of volatile compounds (tar, gases, and char).
- Partial Oxidation: Volatile compounds react with oxygen or steam to produce carbon monoxide (CO), hydrogen (H2), methane (CH4), and other gases.
- Char Gasification: Char residues undergo gasification reactions with carbon dioxide (CO2) or steam (H2O) to produce additional CO and H2.
- Syngas Collection and Cleanup: The raw syngas produced in the fluidized bed gasifier contains impurities such as tar, particulates, sulfur compounds, and ammonia, which must be removed or reduced to acceptable levels. Syngas cleanup technologies such as cyclones, filters, scrubbers, and catalytic converters are used to remove impurities and condition the syngas for downstream utilization.
- Heat Recovery System: Heat generated during the gasification process is recovered and utilized for various purposes, such as preheating biomass feedstocks, generating steam for power generation, or supplying process heat for industrial applications. Heat recovery systems improve overall energy efficiency and reduce the environmental footprint of fluidized bed gasification plants.
Fluidized bed gasification offers several advantages over other gasification technologies, including:
- Uniform Temperature Distribution: Fluidized bed reactors provide excellent heat transfer and temperature control, resulting in uniform temperature distribution throughout the reactor and enhanced gasification performance.
- High Reactor Efficiency: The fluidized bed design allows for continuous mixing and agitation of biomass feedstock particles, maximizing contact with the gasification agent and promoting efficient conversion of biomass into syngas.
- Fuel Flexibility: Fluidized bed gasifiers can accommodate a wide range of biomass feedstocks with varying sizes, moisture contents, and compositions, making them suitable for diverse biomass-to-energy applications.
- Reduced Tar Formation: The turbulent and well-mixed environment in fluidized bed reactors minimizes tar formation and enhances tar cracking and reforming reactions, resulting in cleaner syngas production and reduced downstream cleanup requirements.
Fluidized bed gasification technology has demonstrated its effectiveness and reliability in converting biomass feedstocks into syngas for renewable energy production and waste valorization. Ongoing research and development efforts focused on reactor design optimization, process control, and syngas utilization are essential to further improve the performance and commercial viability of fluidized bed gasification systems for sustainable energy production.
Syngas Composition:
Syngas, short for synthesis gas, is a versatile mixture of gases primarily composed of hydrogen (H2) and carbon monoxide (CO), along with varying amounts of carbon dioxide (CO2), methane (CH4), water vapor (H2O), and other trace gases. The composition of syngas depends on several factors, including the type of biomass feedstock used, the gasification process employed, and the operating conditions of the gasifier. Understanding the composition of syngas is crucial for optimizing gasification processes, designing downstream utilization systems, and assessing the overall performance and environmental impact of biomass-to-energy conversion technologies.
The typical composition of syngas produced from biomass gasification can be summarized as follows:
- Hydrogen (H2): Hydrogen is a key component of syngas and is typically produced through the gasification reactions involving steam (H2O) and carbon (C) in the biomass feedstock. Hydrogen content in syngas can range from 10% to 50% by volume, depending on gasification conditions and feedstock characteristics. High hydrogen content enhances the energy density and combustion properties of syngas, making it suitable for various energy and chemical applications.
- Carbon Monoxide (CO): Carbon monoxide is another major component of syngas and is formed through the partial oxidation of carbonaceous materials in the biomass feedstock. CO content in syngas can range from 15% to 40% by volume and serves as a valuable intermediate for chemical synthesis, fuel production, and combustion processes. High CO content in syngas indicates efficient gasification and carbon conversion.
- Carbon Dioxide (CO2): Carbon dioxide is a byproduct of gasification reactions involving carbon oxidation and gas-phase combustion. CO2 content in syngas typically ranges from 10% to 30% by volume and depends on factors such as the amount of oxygen or air supplied to the gasifier and the degree of carbon conversion. CO2 can be captured and sequestered to reduce greenhouse gas emissions or utilized in various industrial processes.
- Methane (CH4): Methane is a minor component of syngas and is formed through secondary reactions such as methanation and tar reforming. CH4 content in syngas is usually less than 5% by volume but can be higher in gasification systems with low-temperature operation or high methane-forming potential. Methane can be utilized as a fuel or converted into higher-value products through catalytic processes.
- Water Vapor (H2O): Water vapor is present in syngas as a result of steam injection or moisture content in the biomass feedstock. H2O content in syngas varies depending on gasification conditions and can range from 5% to 30% by volume. Water vapor serves as a reactant in gasification reactions and affects the equilibrium composition of syngas.
- Trace Gases: Syngas may contain trace amounts of other gases such as nitrogen (N2), oxygen (O2), hydrogen sulfide (H2S), ammonia (NH3), and volatile organic compounds (VOCs) depending on feedstock composition and gasification conditions. Trace gases can impact syngas quality, combustion performance, and downstream utilization processes and may require removal or treatment for specific applications.
Understanding the composition of syngas is essential for optimizing gasification processes, designing syngas cleanup and conditioning systems, and selecting appropriate utilization pathways for energy production, biofuels synthesis, chemical manufacturing, and other industrial applications. Syngas composition analysis provides valuable insights into gasification performance, carbon conversion efficiency, energy content, and environmental impact, enabling informed decision-making and technology development in the field of biomass-to-energy conversion.
Syngas Cleanup:
Syngas cleanup is a crucial stage in the biomass gasification process that involves the removal or reduction of impurities and contaminants from the raw syngas produced in the gasifier. The cleanup process is essential to ensure the quality, purity, and stability of syngas for downstream utilization in power generation, biofuels production, chemical synthesis, and other industrial applications. Syngas cleanup technologies are designed to remove various impurities, including tar, particulates, sulfur compounds, ammonia, moisture, and trace contaminants, while maintaining the desired composition and properties of the syngas.
Several syngas cleanup technologies and methods are employed to achieve the desired syngas quality:
- Tar Removal: Tar, also known as volatile organic compounds (VOCs), is a complex mixture of hydrocarbons and aromatic compounds formed during biomass gasification. Tar can cause fouling, corrosion, and operational issues in downstream equipment and catalytic converters. Tar removal technologies include:
- Tar Cracking: Thermal or catalytic decomposition of tar molecules at elevated temperatures to break them down into simpler, less harmful compounds.
- Tar Filtration: Passage of syngas through ceramic or metallic filters to physically capture tar particles and condensable compounds.
- Catalytic Tar Reforming: Use of catalysts (e.g., nickel, cobalt, or alkali metals) to catalyze tar cracking reactions and promote the conversion of tar into syngas components.
- Particulate Removal: Particulates, such as ash, char, and fine dust particles, can be present in the raw syngas and can cause erosion, abrasion, and fouling in downstream equipment. Particulate removal technologies include:
- Cyclone Separators: Centrifugal separators that utilize centrifugal force to separate solid particles from the syngas stream based on their mass and size.
- Filter Beds: Porous filter media (e.g., ceramic, metallic, or fibrous materials) that trap and collect solid particles as syngas passes through them.
- Electrostatic Precipitators (ESP): Electrostatically charged plates or wires that attract and capture particulates through electrostatic forces.
- Desulfurization: Sulfur compounds, such as hydrogen sulfide (H2S) and sulfur dioxide (SO2), can be present in the raw syngas and can lead to corrosion, catalyst poisoning, and environmental emissions. Desulfurization technologies include:
- Hydrodesulfurization (HDS): Chemical reaction of sulfur compounds with hydrogen over a catalyst (e.g., cobalt-molybdenum) to convert them into hydrogen sulfide (H2S), which can be removed through scrubbing or adsorption.
- Adsorption: Use of adsorbent materials (e.g., activated carbon, metal oxides) to selectively adsorb sulfur compounds from the syngas stream.
- Ammonia Scrubbing: Ammonia (NH3) can be present in the syngas as a result of nitrogen-containing compounds in the biomass feedstock or as a byproduct of gasification reactions. Ammonia can cause corrosion, catalyst deactivation, and environmental emissions. Ammonia scrubbing technologies involve:
- Wet Scrubbing: Contacting the syngas stream with an aqueous solution (e.g., water or amine solution) to absorb ammonia and form ammonium salts, which can be separated from the liquid phase.
- Moisture Removal: Water vapor (H2O) present in the syngas stream can condense and cause corrosion, reduce heating value, and affect downstream processes. Moisture removal technologies include:
- Cooling and Condensation: Cooling the syngas stream to below its dew point to condense water vapor, followed by separation and removal of the liquid water.
- Absorption: Contacting the syngas stream with a desiccant material (e.g., glycol, silica gel) to selectively absorb water vapor and remove it from the gas stream.
Syngas cleanup is essential for ensuring the efficient and reliable operation of biomass gasification systems and maximizing the performance and environmental benefits of syngas utilization. The selection of syngas cleanup technologies depends on factors such as feedstock composition, gasifier design, syngas quality requirements, and specific application needs. Ongoing research and development efforts are focused on improving the efficiency, reliability, and cost-effectiveness of syngas cleanup technologies to enable the widespread adoption of biomass-to-energy conversion technologies and accelerate the transition to a more sustainable and low-carbon energy system.
Syngas Conditioning:
Syngas conditioning is a critical stage in the biomass gasification process that involves the adjustment and optimization of the composition, temperature, pressure, and cleanliness of the raw syngas produced in the gasifier to meet specific process requirements and maximize its utilization in downstream applications. Syngas conditioning aims to improve syngas quality, stability, and compatibility with various end-use technologies, such as power generation, biofuels production, chemical synthesis, and industrial applications.
Key aspects of syngas conditioning include:
- Gas Composition Adjustment: The composition of raw syngas produced in the gasifier may need to be adjusted to meet the requirements of downstream processes or applications. This may involve modifying the ratios of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other gases in the syngas through catalytic conversion, gas separation, or chemical reactions.
- Tar and Particulate Removal: Raw syngas may contain impurities such as tar, particulates, ash, and contaminants that can interfere with downstream equipment and processes. Syngas conditioning includes tar cracking, particulate removal, and scrubbing to reduce impurity levels and ensure the cleanliness of the syngas stream.
- Temperature Adjustment: The temperature of raw syngas from the gasifier may be too high or too low for certain downstream applications. Syngas conditioning involves cooling or heating the syngas to the desired temperature range using heat exchangers, cooling towers, or direct combustion of syngas.
- Pressure Adjustment: Syngas pressure may need to be adjusted to match the pressure requirements of downstream equipment or processes. Pressure adjustment can be achieved through compression or expansion of the syngas using compressors, expanders, or pressure-reducing valves.
- Moisture Removal: Water vapor (H2O) present in raw syngas can condense and cause corrosion, reduce heating value, and affect downstream processes. Syngas conditioning includes moisture removal through cooling and condensation or absorption using desiccants to ensure the dryness of the syngas stream.
- Gas Purification: Syngas conditioning may involve additional purification steps to remove trace contaminants, sulfur compounds, ammonia, and other impurities that can affect downstream processes or product quality. Gas purification technologies include adsorption, absorption, chemical scrubbing, and catalytic conversion.
Syngas conditioning technologies and methods vary depending on the specific requirements of the gasification process and the intended applications of the syngas. Advanced syngas conditioning systems may incorporate multiple stages of purification, temperature and pressure control, and gas composition adjustment to achieve the desired syngas quality and performance. Optimization of syngas conditioning processes is essential to ensure the efficient and reliable operation of biomass gasification systems and maximize the economic and environmental benefits of syngas utilization. Ongoing research and development efforts are focused on improving syngas conditioning technologies, reducing costs, and enhancing the overall efficiency and sustainability of biomass-to-energy conversion processes.
Syngas Utilization:
Syngas, short for synthesis gas, is a versatile mixture of gases primarily composed of hydrogen (H2) and carbon monoxide (CO), along with varying amounts of carbon dioxide (CO2), methane (CH4), and other trace gases. Syngas produced from biomass gasification processes can be utilized in a wide range of applications across various industries, including power generation, biofuels production, chemical synthesis, and industrial processes. Syngas utilization offers several advantages, including energy diversification, resource efficiency, greenhouse gas reduction, and waste valorization.
Key pathways for syngas utilization include:
- Power Generation:Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity. Gasification-based power generation offers high efficiency and flexibility, allowing for the use of diverse feedstocks and the integration of combined heat and power (CHP) systems to maximize energy recovery and utilization.
- Combined Heat and Power (CHP):Syngas can be used for combined heat and power applications, where waste heat from electricity generation is captured and utilized for heating, cooling, or industrial processes. CHP systems maximize energy efficiency and resource utilization, reducing overall energy consumption and emissions.
- Biofuels Production:Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, biodiesel, and synthetic diesel through catalytic processes such as Fischer-Tropsch synthesis or methanol synthesis. Biofuels produced from syngas offer renewable alternatives to fossil fuels, with lower greenhouse gas emissions and reduced dependence on imported oil.
- Chemical Synthesis:Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols. Chemical synthesis pathways such as ammonia synthesis, methanol synthesis, and hydrocarbon synthesis utilize syngas as a building block for manufacturing a wide range of value-added products.
- Hydrogen Production:Syngas can be converted into hydrogen (H2) through water-gas shift (WGS) reactions or steam reforming processes. Hydrogen produced from syngas serves as a clean and versatile energy carrier for fuel cell vehicles, industrial processes, and chemical manufacturing.
- Synthetic Natural Gas (SNG) Production:Syngas can be upgraded into synthetic natural gas (SNG) through methanation reactions, where CO and H2 are catalytically converted into methane (CH4) and water vapor (H2O). SNG can be injected into natural gas pipelines or used as a transportation fuel.
- Industrial Applications:Syngas can be utilized in various industrial processes such as heat treatment, metal smelting, glass manufacturing, and chemical production. Syngas-based industrial processes offer energy-efficient and environmentally sustainable alternatives to conventional fossil fuel-based technologies.
Syngas utilization plays a crucial role in the transition to a more sustainable and low-carbon energy system by enabling the efficient and environmentally responsible conversion of biomass feedstocks into clean and renewable energy, fuels, and chemicals. Continued research, development, and deployment efforts are essential to advance syngas utilization technologies, improve process efficiency, reduce costs, and enhance the commercial viability of biomass-to-energy conversion systems. Collaboration between industry, academia, and government stakeholders is key to accelerating the adoption of syngas utilization technologies and achieving global energy and climate goals.
Gasification Reactor Design:
Gasification reactor design plays a crucial role in the performance, efficiency, and reliability of biomass gasification processes for the production of syngas. The design of the gasification reactor influences factors such as gasification efficiency, residence time, temperature distribution, heat transfer, feedstock compatibility, tar reduction, and syngas quality. Various types of gasification reactors are employed in biomass gasification systems, each with its own advantages, limitations, and suitability for different feedstocks and applications.
- Fixed-Bed Gasifier:
- In a fixed-bed gasifier, biomass feedstock is fed into a stationary reactor bed, typically filled with a bed of inert material (e.g., sand, char, or ceramic beads).
- Gasification reactions occur as the biomass moves downward through the reactor bed, undergoing pyrolysis, oxidation, and gasification.
- Fixed-bed gasifiers offer simplicity, reliability, and scalability, making them suitable for small-scale and decentralized biomass gasification applications.
- However, fixed-bed gasifiers may suffer from limited heat transfer, uneven temperature distribution, and bed agglomeration issues, particularly with high-moisture or high-ash feedstocks.
- Fluidized Bed Gasifier:
- In a fluidized bed gasifier, biomass feedstock is suspended and fluidized by a stream of gas (e.g., air, oxygen, steam) flowing upward through the reactor bed.
- The fluidized bed of biomass particles promotes excellent mixing, heat transfer, and gas-solid contact, leading to efficient gasification reactions.
- Fluidized bed gasifiers offer high gasification efficiency, uniform temperature distribution, and tolerance to variations in feedstock size, moisture content, and composition.
- However, fluidized bed gasifiers may require complex gas-solid separation systems, have higher operating costs, and may be prone to erosion and abrasion of reactor components.
- Entrained-Flow Gasifier:
- In an entrained-flow gasifier, finely ground biomass feedstock is entrained by a high-velocity stream of gas (e.g., oxygen, steam) and injected into a reaction chamber.
- Gasification reactions occur in a turbulent, high-temperature environment, resulting in rapid conversion of biomass into syngas.
- Entrained-flow gasifiers offer high gasification rates, high thermal efficiency, and flexibility in feedstock selection.
- However, entrained-flow gasifiers require robust refractory materials, precise control of gas flow and temperature, and may produce tar and particulate emissions that require downstream cleanup.
- Hybrid Gasifier:
- Hybrid gasifiers combine features of different gasification reactor types to leverage their respective advantages and overcome limitations.
- For example, hybrid gasifiers may combine a fixed-bed or fluidized bed for biomass pyrolysis and partial oxidation with an entrained-flow reactor for complete gasification of char and tar.
- Hybrid gasifiers aim to achieve high gasification efficiency, improved tar reduction, and enhanced process flexibility for a wide range of feedstocks and applications.
Gasification reactor design considerations include reactor geometry, size, shape, configuration, material selection, insulation, heating methods, gas distribution, mixing, residence time, and process control strategies. Advanced computational modeling, simulation, and experimental testing techniques are employed to optimize gasification reactor designs and improve performance, reliability, and cost-effectiveness. Ongoing research and development efforts are focused on advancing gasification reactor technologies, enhancing process efficiency, reducing emissions, and enabling the widespread adoption of biomass-to-energy conversion systems for sustainable energy production.
Syngas Tar Removal:
Syngas tar removal is a critical process in biomass gasification systems aimed at reducing the concentration of tar compounds in the raw syngas stream. Tar, also known as volatile organic compounds (VOCs), is a complex mixture of hydrocarbons and aromatic compounds produced during the pyrolysis and thermal decomposition of biomass feedstocks. Tar compounds can cause fouling, corrosion, and operational issues in downstream equipment and catalysts, leading to reduced efficiency and reliability of syngas utilization processes.
Several methods and technologies are employed for syngas tar removal:
- Tar Cracking:
- Tar cracking involves the thermal or catalytic decomposition of tar molecules at elevated temperatures to break them down into simpler, less harmful compounds.
- Thermal tar cracking occurs naturally at high temperatures (> 800°C) in the gasification reactor and downstream equipment, promoting the conversion of tar into syngas components (e.g., CO, H2, CH4).
- Catalytic tar cracking utilizes catalyst materials (e.g., nickel, cobalt, alkali metals) to accelerate tar decomposition reactions and enhance tar conversion efficiency at lower temperatures.
- Tar Filtration:
- Tar filtration involves passing the raw syngas stream through porous filter media (e.g., ceramic or metallic filters) to physically capture and remove tar particles and condensable compounds.
- Filter beds can be designed with different pore sizes and configurations to efficiently trap tar particles while allowing syngas to pass through.
- Tar filtration is effective for removing coarse tar particles but may require periodic cleaning or replacement of filter elements to maintain optimal performance.
- Catalytic Tar Reforming:
- Catalytic tar reforming utilizes catalysts to promote the cracking and reforming of tar molecules into simpler hydrocarbons and syngas components.
- Reforming reactions occur at lower temperatures and with higher selectivity compared to thermal cracking, reducing energy consumption and minimizing the formation of undesirable byproducts.
- Catalyst materials and reactor configurations are optimized to maximize tar conversion efficiency and minimize catalyst deactivation due to fouling or poisoning.
- Tar Scrubbing:
- Tar scrubbing involves contacting the raw syngas stream with a liquid scrubbing solution to absorb tar compounds and condensable vapors.
- Scrubbing solutions may include water, oil, solvents, or chemical additives that selectively dissolve tar components and separate them from the syngas stream.
- Tar scrubbing can effectively remove tar contaminants but may require additional downstream processing steps to recover and recycle the scrubbing solution.
- Tar Decomposition Catalysts:
- Tar decomposition catalysts are specifically designed catalyst materials that promote the cracking and reforming of tar compounds into lighter hydrocarbons and syngas components.
- Catalysts can be deployed in various configurations, including fixed-bed reactors, fluidized bed reactors, or integrated into gasification reactor systems.
- Tar decomposition catalysts enhance tar conversion efficiency, reduce tar emissions, and improve the overall performance of biomass gasification processes.
Syngas tar removal technologies are essential for ensuring the reliability, efficiency, and environmental sustainability of biomass gasification systems. Effective tar removal minimizes the risk of equipment fouling, improves syngas quality, and enhances the economic viability of syngas utilization pathways for power generation, biofuels production, chemical synthesis, and industrial applications. Ongoing research and development efforts are focused on advancing tar removal technologies, optimizing process integration, and reducing costs to enable the widespread adoption of biomass-to-energy conversion systems for sustainable energy production.
Gasification
Gasification is a thermochemical conversion process that transforms carbon-containing feedstocks, such as biomass, coal, or waste materials, into a gaseous mixture known as syngas or synthesis gas. This process occurs under controlled conditions of temperature, pressure, and oxygen supply, typically in the absence of complete combustion. Gasification represents a versatile and efficient approach to convert diverse feedstocks into a valuable energy carrier with numerous applications.
The gasification process involves several key steps:
- Feedstock Preparation: The feedstock, which can range from wood chips and agricultural residues to coal and municipal solid waste, undergoes pretreatment to achieve the desired size, moisture content, and composition. This may involve drying, shredding, or pelletizing the feedstock to enhance its suitability for gasification.
- Gasification Reaction: The prepared feedstock is introduced into the gasification reactor, where it undergoes chemical reactions in the presence of a controlled amount of oxygen, steam, or a combination of both. These reactions typically include pyrolysis (thermal decomposition), combustion (partial oxidation), and gasification (conversion of carbon to syngas constituents).
- Syngas Production: The primary product of the gasification process is syngas, a mixture of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other trace gases. The composition of syngas depends on factors such as the feedstock type, gasification temperature, residence time, and gasification agent.
- Tar and Particulate Removal: During gasification, tar compounds and particulate matter may be formed as byproducts of incomplete combustion and thermal decomposition. These impurities can foul equipment, reduce gas quality, and pose environmental and health risks. Therefore, syngas undergoes purification steps, such as tar cracking, filtration, or scrubbing, to remove these contaminants and improve syngas quality.
- Syngas Utilization: The produced syngas can be utilized in various applications, including power generation, biofuels production, chemical synthesis, and industrial processes. Depending on the specific requirements and desired end products, syngas may undergo further processing, such as catalytic conversion, reforming, or upgrading, to tailor its composition and properties for different applications.
Gasification offers several advantages compared to conventional combustion-based technologies:
- Fuel Flexibility: Gasification can utilize a wide range of feedstocks, including biomass, coal, municipal solid waste, and industrial residues, providing flexibility and resilience in energy supply.
- Energy Efficiency: Gasification processes can achieve high energy conversion efficiencies, especially when integrated with combined heat and power (CHP) systems, where waste heat is utilized for heating or industrial processes.
- Emissions Reduction: Gasification can produce syngas with lower emissions of greenhouse gases, particulates, and sulfur compounds compared to conventional combustion processes, contributing to environmental sustainability and air quality improvement.
- Resource Recovery: Gasification facilitates the recovery of energy and valuable byproducts from waste materials, promoting resource conservation and waste valorization.
Gasification technology continues to evolve with ongoing research and development efforts aimed at improving efficiency, reducing costs, and expanding the range of feedstocks and applications. The versatility, efficiency, and environmental benefits of gasification make it a promising technology for sustainable energy production and resource management in the transition towards a low-carbon economy.
Biomass
Biomass refers to organic materials derived from living or recently living organisms, such as plants, trees, agricultural residues, algae, and organic waste. It is a renewable and abundant energy resource that can be converted into various forms of energy, including heat, electricity, and biofuels, through processes such as combustion, gasification, pyrolysis, and biochemical conversion. Biomass plays a significant role in the transition to a sustainable and low-carbon energy system due to its carbon-neutral nature and potential to replace fossil fuels in energy production and other applications.
The utilization of biomass for energy dates back to ancient times when humans used wood and other biomass materials for cooking, heating, and lighting. Today, biomass remains a vital energy resource, contributing to the global energy mix and providing numerous environmental, economic, and social benefits:
- Renewable Energy Source: Biomass is considered renewable because it can be replenished through natural processes such as photosynthesis and regrowth of plants. Unlike fossil fuels, which are finite and non-renewable, biomass can be sustainably managed and harvested to meet current and future energy needs without depleting natural resources.
- Carbon Neutrality: Biomass combustion and conversion processes release carbon dioxide (CO2) into the atmosphere, but the carbon emitted is part of the natural carbon cycle. Since biomass crops absorb CO2 during their growth phase, they effectively offset the CO2 emissions from their combustion, making biomass energy carbon-neutral over its lifecycle. This carbon neutrality distinguishes biomass from fossil fuels, which release additional CO2 stored underground and contribute to global warming.
- Waste Management and Recycling: Biomass energy production provides an opportunity to divert organic waste materials, such as agricultural residues, forestry residues, and food waste, from landfills and incinerators. By converting these waste materials into energy, biomass helps reduce methane emissions from decomposing organic matter and minimizes environmental pollution and landfill space requirements.
- Rural Development and Economic Opportunities: Biomass energy production can stimulate economic growth and create employment opportunities, particularly in rural areas where biomass resources are abundant. Biomass cultivation, harvesting, processing, and conversion activities generate jobs along the supply chain, supporting local economies and fostering sustainable development.
- Energy Security and Diversification: Biomass offers a decentralized and distributed energy source that can be produced and utilized locally, reducing reliance on imported fossil fuels and enhancing energy security. Biomass resources are available in diverse forms and locations worldwide, providing opportunities for energy diversification and resilience against supply disruptions.
- Bioenergy Applications: Biomass can be converted into various forms of bioenergy, including solid biomass (e.g., wood pellets, agricultural residues), liquid biofuels (e.g., ethanol, biodiesel), biogas (produced through anaerobic digestion of organic waste), and syngas (produced through biomass gasification). These bioenergy sources can be used for heating, electricity generation, transportation fuels, and industrial processes, offering versatile and sustainable alternatives to fossil fuels.
Overall, biomass represents a valuable renewable energy resource with significant potential to contribute to climate change mitigation, environmental protection, and sustainable development. However, effective biomass utilization requires careful consideration of environmental, social, and economic factors, as well as technological advancements and policy support to maximize its benefits while minimizing potential risks and trade-offs.
Syngas
Syngas, short for synthesis gas, is a versatile mixture of gases primarily composed of hydrogen (H2) and carbon monoxide (CO), along with varying amounts of carbon dioxide (CO2), methane (CH4), and other trace gases. It is produced through the gasification of carbon-containing feedstocks such as biomass, coal, or natural gas, typically in the presence of a controlled amount of oxygen, steam, or a combination of both. Syngas serves as a valuable intermediate in various energy conversion processes and industrial applications due to its combustible nature and chemical reactivity.
The composition of syngas can vary depending on factors such as the feedstock type, gasification process conditions, and gasification technology employed. However, the primary constituents of syngas include:
- Hydrogen (H2): Hydrogen is a key component of syngas and serves as a clean and versatile energy carrier in various applications. It can be used directly as a fuel for heating, power generation, or transportation, or as a feedstock for chemical synthesis processes such as ammonia production, methanol synthesis, and hydrocarbon reforming.
- Carbon Monoxide (CO): Carbon monoxide is another important component of syngas and is often used as a reducing agent in chemical processes such as steel production, methanol synthesis, and Fischer-Tropsch synthesis. It can also be converted into hydrogen through water-gas shift reactions for further utilization in fuel cells or ammonia synthesis.
- Carbon Dioxide (CO2): Carbon dioxide is a byproduct of the gasification process and is typically present in syngas at varying concentrations. While CO2 is considered a greenhouse gas and contributes to global warming, it can also be captured and sequestered to reduce emissions or utilized in applications such as enhanced oil recovery, carbonation of concrete, or algae cultivation for biofuel production.
- Methane (CH4): Methane is a minor component of syngas and is typically produced through secondary reactions such as methanation or tar reforming. While methane can be utilized as a fuel for heating or power generation, its presence in syngas may need to be minimized to avoid undesirable combustion properties or emissions.
Syngas finds numerous applications across various industries, including:
- Power Generation: Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity. Combined heat and power (CHP) systems can maximize energy efficiency by utilizing waste heat for heating or industrial processes.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, biodiesel, and synthetic diesel through catalytic processes such as Fischer-Tropsch synthesis or methanol synthesis.
- Chemical Synthesis: Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols.
- Hydrogen Production: Syngas can be converted into hydrogen through water-gas shift reactions or steam reforming processes, providing a clean and versatile energy carrier for fuel cell vehicles, industrial processes, and chemical manufacturing.
Overall, syngas represents a valuable intermediate in the utilization of carbon-containing feedstocks for energy production and industrial applications. Its versatility, combustibility, and chemical reactivity make it an essential component of the transition to a more sustainable and low-carbon energy system. Continued research and development efforts are focused on advancing syngas production technologies, improving process efficiency, and expanding its range of applications to address global energy and environmental challenges.
Reactor
In the context of gasification and biomass energy production, a reactor refers to a vessel or system designed to facilitate the conversion of feedstock into useful products such as syngas, biofuels, or chemicals through controlled chemical reactions. Reactors play a central role in various biomass conversion processes, including gasification, pyrolysis, fermentation, and catalytic conversion, by providing the necessary conditions for biomass transformation while optimizing process efficiency, product yields, and quality.
The design and operation of biomass reactors are influenced by several factors, including feedstock characteristics, desired products, reaction kinetics, thermodynamics, heat and mass transfer phenomena, and process requirements. Reactor technologies range from simple batch reactors to complex continuous-flow systems, each offering distinct advantages and limitations depending on the specific application and scale of operation.
Here are some common types of reactors used in biomass conversion processes:
- Fixed-Bed Reactor:
- In a fixed-bed reactor, biomass feedstock is loaded into a stationary bed within the reactor vessel, where it undergoes conversion reactions under controlled temperature, pressure, and gas flow conditions.
- Fixed-bed reactors offer simplicity, reliability, and ease of operation, making them suitable for small-scale gasification, pyrolysis, and biochar production applications.
- However, fixed-bed reactors may suffer from limited heat and mass transfer, uneven temperature distribution, and bed agglomeration issues, particularly with high-moisture or high-ash feedstocks.
- Fluidized Bed Reactor:
- In a fluidized bed reactor, biomass feedstock is suspended and fluidized by a stream of gas (e.g., air, steam) flowing upward through the reactor vessel. The fluidized bed enhances mixing, heat transfer, and gas-solid contact, leading to efficient biomass conversion.
- Fluidized bed reactors offer high reaction rates, uniform temperature distribution, and tolerance to variations in feedstock properties, making them suitable for biomass gasification, pyrolysis, and combustion processes.
- However, fluidized bed reactors may require complex gas-solid separation systems and are susceptible to erosion and abrasion of reactor components.
- Entrained-Flow Reactor:
- In an entrained-flow reactor, finely ground biomass feedstock is entrained by a high-velocity stream of gas (e.g., oxygen, steam) and injected into a reaction chamber, where rapid conversion reactions occur in a turbulent, high-temperature environment.
- Entrained-flow reactors offer high reaction rates, high thermal efficiency, and flexibility in feedstock selection, making them suitable for biomass gasification and fast pyrolysis applications.
- However, entrained-flow reactors require robust refractory materials and precise control of gas flow and temperature to maintain stable operation.
- Hybrid Reactor:
- Hybrid reactors combine features of different reactor types to leverage their respective advantages and overcome limitations. For example, hybrid gasifiers may combine a fixed-bed or fluidized bed for biomass pyrolysis and partial oxidation with an entrained-flow reactor for complete gasification of char and tar.
- Hybrid reactors aim to achieve high conversion efficiency, improved product quality, and enhanced process flexibility for a wide range of biomass conversion applications.
Reactor design considerations include reactor geometry, size, shape, configuration, material selection, insulation, heating methods, gas distribution, mixing, residence time, and process control strategies. Advanced computational modeling, simulation, and experimental testing techniques are employed to optimize reactor designs and improve performance, reliability, and cost-effectiveness. Ongoing research and development efforts are focused on advancing reactor technologies, enhancing process efficiency, and enabling the widespread adoption of biomass-to-energy conversion systems for sustainable energy production.
Feedstock
Feedstock refers to the raw material or biomass resource used as a primary input in various conversion processes to produce energy, fuels, chemicals, or other value-added products. In the context of biomass energy production, feedstock encompasses a wide range of organic materials derived from biological sources, including plants, trees, crops, agricultural residues, forestry residues, algae, and organic waste.
The selection of feedstock plays a critical role in determining the overall efficiency, economics, and environmental sustainability of biomass conversion processes. Different feedstocks have unique characteristics, including composition, moisture content, energy density, ash content, and availability, which influence their suitability for specific applications and conversion technologies.
Here are some common types of biomass feedstocks used in biomass energy production:
- Woody Biomass:
- Woody biomass includes trees, branches, bark, and wood residues from forestry operations, sawmills, and timber processing industries.
- Woody biomass is a widely available feedstock with high energy density and relatively low moisture content, making it suitable for combustion, gasification, and biochar production.
- Agricultural Residues:
- Agricultural residues are byproducts of crop cultivation and harvesting processes, such as straw, stalks, husks, shells, and bagasse.
- Agricultural residues are abundant, widely distributed, and often available at low cost, making them attractive feedstocks for bioenergy production, particularly in rural areas.
- Energy Crops:
- Energy crops are specifically grown for biomass energy production and include fast-growing plants such as switchgrass, miscanthus, willow, and poplar.
- Energy crops offer high biomass yields, rapid growth rates, and efficient use of land and resources, making them promising feedstocks for biofuels and biopower production.
- Municipal Solid Waste (MSW):
- Municipal solid waste consists of household, commercial, and industrial waste materials, including paper, cardboard, plastics, food scraps, and yard waste.
- MSW can be processed through mechanical or biological treatments to recover organic fractions suitable for anaerobic digestion, composting, or thermal conversion into energy.
- Algal Biomass:
- Algal biomass refers to microalgae or macroalgae cultivated in aquatic environments for biofuel production, wastewater treatment, carbon capture, and other applications.
- Algae offer high growth rates, efficient carbon fixation, and the ability to thrive in diverse environments, making them promising feedstocks for biofuel and bioproducts production.
The choice of feedstock depends on factors such as feedstock availability, cost, sustainability, environmental impact, conversion technology requirements, and end-product specifications. Sustainable feedstock sourcing practices, including land management, crop rotation, agroforestry, and waste-to-energy initiatives, are essential to ensure the long-term viability and environmental integrity of biomass energy production. Additionally, advancements in feedstock preprocessing, densification, storage, and logistics are needed to improve feedstock quality, handling, and supply chain efficiency for biomass conversion processes.
Tar
Tar, also known as volatile organic compounds (VOCs) or tars, refers to a complex mixture of organic compounds produced during the thermal decomposition and gasification of biomass feedstocks. Tars are formed as byproducts of pyrolysis, combustion, and gasification reactions, particularly at elevated temperatures (>400°C), when organic molecules undergo incomplete conversion into gases such as carbon monoxide (CO), hydrogen (H2), methane (CH4), and carbon dioxide (CO2).
The composition and properties of tar vary depending on factors such as the feedstock type, gasification conditions, reactor design, residence time, and gasification technology employed. Tars consist of a wide range of hydrocarbons, oxygenated compounds, nitrogen-containing compounds, and trace elements, with molecular weights ranging from light volatiles to heavy, high-molecular-weight compounds.
Tars pose several challenges in biomass gasification and syngas utilization processes:
- Equipment Fouling: Tar compounds can condense and deposit on the surfaces of gasification reactor walls, heat exchangers, syngas pipelines, and downstream equipment, leading to fouling, plugging, and corrosion issues. Tar deposits reduce heat transfer efficiency, increase pressure drop, and degrade equipment performance, requiring frequent maintenance and cleaning to restore operation.
- Catalyst Deactivation: Tars can poison or deactivate catalyst materials used in downstream syngas cleanup, conditioning, and utilization processes. Catalyst deactivation occurs due to adsorption, deposition, and coking of tar compounds on the catalyst surface, inhibiting active sites and reducing catalytic activity and selectivity.
- Syngas Contamination: Tars present in syngas streams can contaminate downstream utilization pathways such as engines, turbines, fuel cells, and chemical reactors, leading to decreased efficiency, increased emissions, and equipment damage. Tar contaminants may cause fouling, erosion, corrosion, and catalyst poisoning, affecting system reliability and performance.
Several methods and technologies are employed for tar removal and mitigation in biomass gasification systems:
- Tar Cracking: Thermal or catalytic decomposition of tar molecules at high temperatures (>800°C) promotes the conversion of heavy tar compounds into lighter gases such as CO, H2, and methane (CH4). Tar cracking occurs naturally in the gasification reactor and downstream equipment, but additional tar cracking catalysts or reactors may be employed to enhance tar conversion efficiency.
- Tar Filtration: Passage of the raw syngas stream through porous filter media (e.g., ceramic or metallic filters) captures and removes tar particles and condensable compounds. Tar filtration is effective for removing coarse tar particles but may require periodic cleaning or replacement of filter elements.
- Catalytic Tar Reforming: Catalyst materials promote the cracking and reforming of tar compounds into simpler hydrocarbons and syngas components at lower temperatures and with higher selectivity. Tar reforming catalysts enhance tar conversion efficiency and minimize catalyst deactivation due to fouling or poisoning.
- Tar Scrubbing: Contacting the raw syngas stream with a liquid scrubbing solution (e.g., water, oil, solvents) absorbs tar compounds and condensable vapors. Tar scrubbing effectively removes tar contaminants but may require additional downstream processing steps to recover and recycle the scrubbing solution.
Tar removal technologies are essential for ensuring the reliability, efficiency, and environmental sustainability of biomass gasification systems. Effective tar removal minimizes equipment fouling, improves syngas quality, and enhances the economic viability of syngas utilization pathways for power generation, biofuels production, chemical synthesis, and industrial applications. Ongoing research and development efforts are focused on advancing tar removal technologies, optimizing process integration, and reducing costs to enable the widespread adoption of biomass-to-energy conversion systems for sustainable energy production.
Particulate:
Particulate matter (PM), often referred to simply as particulate, is a complex mixture of tiny solid particles and liquid droplets suspended in air. In the context of biomass gasification and energy production, particulate matter is generated during the thermal conversion of biomass feedstocks and can include a variety of organic and inorganic compounds, such as ash, char, soot, tar, and aerosols.
The formation and characteristics of particulate matter depend on various factors, including the composition and properties of the biomass feedstock, gasification process conditions (e.g., temperature, pressure, residence time), reactor design, gas composition, and combustion kinetics. Particulate matter can vary in size, shape, density, chemical composition, and reactivity, influencing its behavior, transport, and environmental impacts.
Particulate matter poses several challenges and considerations in biomass gasification and energy production systems:
- Air Quality and Health Effects: Inhalation of particulate matter can pose significant health risks, particularly for vulnerable populations such as children, the elderly, and individuals with respiratory or cardiovascular conditions. Fine particulate matter (PM2.5) can penetrate deep into the lungs and bloodstream, causing respiratory problems, cardiovascular diseases, and premature mortality. Therefore, minimizing particulate emissions from biomass gasification processes is essential to protect public health and ensure compliance with air quality regulations.
- Environmental Impacts: Particulate matter emissions from biomass gasification can contribute to environmental pollution, including smog formation, visibility impairment, acid deposition, and ecosystem damage. Particulate matter can also serve as a carrier for hazardous pollutants such as heavy metals, polycyclic aromatic hydrocarbons (PAHs), and dioxins, which pose risks to human health and the environment.
- Equipment Fouling: Particulate matter can deposit on the surfaces of gasification reactor walls, heat exchangers, syngas pipelines, and downstream equipment, leading to fouling, plugging, and corrosion issues. Particulate deposits reduce heat transfer efficiency, increase pressure drop, and degrade equipment performance, necessitating frequent maintenance and cleaning to restore operation.
- Syngas Quality and Utilization: Particulate matter present in syngas streams can contaminate downstream utilization pathways such as engines, turbines, fuel cells, and chemical reactors, leading to decreased efficiency, increased emissions, and equipment damage. Particulate contaminants may cause fouling, erosion, corrosion, and catalyst deactivation, affecting system reliability and performance.
To mitigate the impacts of particulate matter emissions from biomass gasification processes, various control measures and technologies are employed:
- Particulate Removal Systems: Mechanical collectors, such as cyclones, electrostatic precipitators (ESPs), fabric filters (baghouses), and wet scrubbers, are used to capture and remove particulate matter from gas streams. These systems rely on principles such as inertia, electrostatic attraction, filtration, and impaction to separate particles from the gas phase.
- Gasification Process Optimization: Adjusting gasification process parameters, such as temperature, residence time, and gas flow rates, can influence particulate formation and emissions. Optimizing process conditions can minimize the generation of fine particulate matter and promote the conversion of volatile organic compounds into syngas constituents.
- Fuel Preparation and Handling: Preprocessing biomass feedstocks through drying, grinding, pelletizing, or densification can reduce moisture content, particle size, and variability, leading to more uniform combustion or gasification behavior and lower particulate emissions.
- Advanced Gasification Technologies: Advanced gasification technologies, such as fluidized bed reactors, entrained-flow reactors, and hybrid gasification systems, offer improved mixing, heat transfer, and gas-solid contact, leading to enhanced combustion efficiency and reduced particulate emissions.
- Particulate Monitoring and Control: Continuous monitoring of particulate emissions from biomass gasification systems is essential to assess compliance with regulatory standards, optimize process performance, and identify opportunities for emission reduction. Real-time particulate monitoring technologies, such as optical instruments, laser-based sensors, and electrochemical sensors, enable rapid detection and response to changes in particulate levels.
Overall, minimizing particulate matter emissions from biomass gasification processes is essential to protect human health, safeguard the environment, and ensure the sustainability of biomass energy production. Effective particulate control measures and technologies enable the development of cleaner and more efficient biomass gasification systems for sustainable energy generation and resource utilization.
Fluidized Bed Reactor:
A fluidized bed reactor is a type of reactor used in various chemical, petrochemical, and energy production processes, including biomass gasification, combustion, pyrolysis, and catalytic reactions. It operates on the principle of fluidization, where a bed of solid particles is suspended and behaves like a fluid when subjected to a flow of gas. In biomass gasification, fluidized bed reactors are widely used due to their ability to handle a wide range of feedstocks, high reaction rates, efficient heat and mass transfer, and flexibility in operation.
Here’s how a fluidized bed reactor works in the context of biomass gasification:
- Bed Material: The reactor contains a bed of inert solid particles, typically sand, alumina, or silica, which serves as the support medium for biomass feedstock. The particle size and density are carefully selected to achieve fluidization under the desired operating conditions.
- Gas Flow: A gas, typically air, oxygen, steam, or a mixture of gases, is introduced into the bottom of the reactor and passes through the bed of solid particles. The upward flow of gas fluidizes the bed, causing the particles to behave like a fluid with characteristics similar to boiling water.
- Biomass Feedstock Injection: Biomass feedstock, such as wood chips, agricultural residues, or energy crops, is injected into the fluidized bed reactor either directly or through a feeding mechanism. The biomass particles mix with the fluidized bed and undergo thermal decomposition and gasification reactions in the presence of the gasification agent.
- Reaction Zone: Within the fluidized bed reactor, biomass feedstock undergoes a series of complex thermochemical reactions, including drying, pyrolysis, combustion, and gasification. These reactions occur at elevated temperatures (typically 700°C to 1000°C) and in the presence of limited oxygen or steam to produce a mixture of gases known as syngas.
- Heat Transfer: Heat generated by exothermic gasification reactions is transferred to the surrounding bed material and biomass particles, promoting thermal decomposition and gasification. The fluidized bed configuration enhances heat transfer efficiency by ensuring intimate contact between the solid particles and the reacting gases.
- Syngas Production: The primary product of biomass gasification in a fluidized bed reactor is syngas, a mixture of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other trace gases. The composition of syngas depends on factors such as the biomass feedstock, gasification conditions, and reactor design.
- Gas-Solid Separation: After gasification reactions occur, the syngas rises through the fluidized bed and exits the reactor through an outlet at the top. Particulate matter, ash, and unreacted char particles are entrained in the gas stream and may be separated using cyclones, filters, or other gas-solid separation devices before further processing or utilization.
Fluidized bed reactors offer several advantages for biomass gasification applications:
- High Reaction Rates: Fluidized bed reactors provide excellent mixing and gas-solid contact, leading to rapid biomass conversion and high reaction rates.
- Flexibility: Fluidized bed reactors can accommodate a wide range of biomass feedstocks, including low-quality or high-moisture materials, without significant changes to the reactor design.
- Good Heat Transfer: The fluidized bed configuration enhances heat transfer efficiency, allowing for effective thermal decomposition and gasification of biomass feedstock.
- Reduced Tar Formation: Fluidized bed reactors promote tar cracking and reforming reactions, minimizing tar formation and improving syngas quality compared to other gasification technologies.
However, fluidized bed reactors also present some challenges, including the potential for bed agglomeration, particle attrition, elutriation of fine particles, and erosion of reactor components. Proper reactor design, operation, and maintenance are essential to mitigate these issues and ensure the reliable and efficient operation of fluidized bed biomass gasification systems. Ongoing research and development efforts are focused on advancing fluidized bed reactor technologies, optimizing process performance, and expanding their applications in sustainable energy production.
Gasification
Gasification is a thermochemical conversion process that transforms carbonaceous materials, such as biomass, coal, or organic waste, into a mixture of gases known as syngas (synthesis gas) through partial oxidation in a controlled environment. This process involves the reaction of the carbonaceous feedstock with a gasification agent, typically air, oxygen, steam, or a combination thereof, at elevated temperatures (> 700°C) and pressure. Gasification offers a versatile and efficient means of converting a wide range of feedstocks into a valuable energy carrier and chemical precursor.
Here’s how the gasification process works:
- Feedstock Preparation: The carbonaceous feedstock, such as biomass (e.g., wood chips, agricultural residues) or coal, is first prepared by sizing, drying, and grinding to facilitate handling, transportation, and feeding into the gasification reactor. Feedstock preparation may also involve removing contaminants and moisture to improve gasification efficiency.
- Gasification Reactor: The prepared feedstock is fed into a gasification reactor, which can take various forms depending on the specific technology employed. Common gasification reactor designs include fixed-bed reactors, fluidized bed reactors, entrained-flow reactors, and hybrid configurations. The reactor provides the necessary conditions for the thermochemical conversion of the feedstock into syngas, including elevated temperatures, controlled oxygen or steam supply, and sufficient residence time.
- Thermochemical Reactions: Within the gasification reactor, the carbonaceous feedstock undergoes a series of complex thermochemical reactions in the presence of the gasification agent. These reactions include:
- Drying: Removal of moisture from the feedstock at temperatures below 100°C.
- Pyrolysis: Thermal decomposition of organic matter in the absence of oxygen to produce volatile gases, tar, and char.
- Combustion: Oxidation of char and volatile gases in the presence of oxygen to release heat and produce carbon dioxide (CO2) and water vapor (H2O).
- Gasification: Reaction of carbon (C) with steam (H2O) or carbon dioxide (CO2) to produce hydrogen (H2) and carbon monoxide (CO) via the water-gas shift reaction or the carbon-gas shift reaction, respectively.
- Syngas Production: The primary product of gasification is syngas, a mixture of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other trace gases. The composition of syngas depends on factors such as the feedstock type, gasification conditions, and reactor design. Syngas has a variety of potential applications, including power generation, heat production, biofuels synthesis, chemical manufacturing, and as a feedstock for hydrogen production.
- Gas Cleaning and Conditioning: The raw syngas produced from the gasification reactor typically contains impurities such as tar, particulate matter, sulfur compounds, and trace metals, which must be removed or reduced to acceptable levels before further utilization. Gas cleaning and conditioning processes may include filtration, scrubbing, catalytic conversion, and gas cooling to improve syngas quality and stability.
- Syngas Utilization: Cleaned and conditioned syngas can be utilized in various downstream processes and applications, including:
- Power Generation: Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity and heat in combined heat and power (CHP) systems.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, synthetic diesel, and Fischer-Tropsch fuels through catalytic synthesis processes.
- Chemical Synthesis: Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols.
Gasification offers several advantages over traditional combustion technologies, including higher energy efficiency, lower emissions, and greater fuel flexibility. It enables the conversion of a wide range of feedstocks, including low-grade and waste materials, into valuable energy products, contributing to resource utilization, waste management, and renewable energy production goals. Ongoing research and development efforts are focused on advancing gasification technologies, improving process efficiency, and expanding the range of feedstocks and applications for sustainable energy production.
Syngas
Syngas, short for synthesis gas, is a mixture of gases primarily composed of hydrogen (H2) and carbon monoxide (CO), along with varying amounts of carbon dioxide (CO2), methane (CH4), water vapor (H2O), and trace impurities. It is produced through the gasification of carbonaceous feedstocks such as biomass, coal, natural gas, or organic waste in the presence of a gasification agent, typically air, oxygen, steam, or a combination thereof. Syngas serves as a versatile intermediate product that can be utilized in a wide range of industrial processes and energy applications.
Here are some key characteristics and applications of syngas:
- Composition: The composition of syngas varies depending on factors such as the type of feedstock, gasification process conditions, and reactor design. Typically, syngas contains hydrogen (H2) and carbon monoxide (CO) as the primary components, with hydrogen content ranging from 15% to 50% by volume and carbon monoxide content ranging from 20% to 40% by volume. The ratio of hydrogen to carbon monoxide, known as the H2/CO ratio, influences the potential applications of syngas.
- Energy Content: Syngas has a high energy content due to the presence of hydrogen and carbon monoxide, which can be combusted or converted into other fuels and chemicals through various synthesis processes. The calorific value of syngas depends on its composition and typically ranges from 4 to 12 megajoules per cubic meter (MJ/m³), depending on the hydrogen content.
- Applications:
- Power Generation: Syngas can be used as a fuel for power generation in gas turbines, reciprocating engines, or fuel cells. Combined heat and power (CHP) systems utilize syngas to produce both electricity and heat, improving overall energy efficiency.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, synthetic diesel, and Fischer-Tropsch fuels through catalytic synthesis processes. These biofuels can be used as transportation fuels or blended with conventional fuels to reduce greenhouse gas emissions.
- Chemical Synthesis: Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols. These chemicals are used in fertilizer production, petrochemical manufacturing, hydrogen production, and other industrial processes.
- Hydrogen Production: Syngas can be converted into pure hydrogen (H2) through processes such as water-gas shift reaction (WGSR) or steam methane reforming (SMR). Hydrogen is a clean and versatile energy carrier used in fuel cells, ammonia production, refining, and chemical synthesis.
- Carbon Capture and Utilization (CCU): Syngas can serve as a feedstock for carbon capture and utilization (CCU) processes, where carbon dioxide (CO2) is captured from syngas streams and converted into value-added products such as synthetic fuels, chemicals, and building materials.
Syngas production can be tailored to meet specific requirements for different applications by adjusting gasification process parameters, such as feedstock composition, gasification agent ratio, temperature, pressure, and residence time. Advances in gasification technology, catalyst development, and process optimization continue to expand the range of syngas-based applications and contribute to the transition toward cleaner and more sustainable energy systems.
Biomass Gasification Plant
A biomass gasification plant is a facility that converts biomass feedstocks into syngas (synthesis gas) through the gasification process. These plants play a crucial role in the production of renewable energy, biofuels, and biochemicals by utilizing organic materials such as wood, agricultural residues, energy crops, and organic waste as feedstocks. Biomass gasification plants typically consist of several key components and processes designed to efficiently convert biomass into syngas while minimizing environmental impacts.
Here are the main components and processes involved in a biomass gasification plant:
- Feedstock Handling and Preparation: The biomass feedstock, sourced from forestry operations, agriculture, or organic waste streams, is transported to the gasification plant and undergoes various preparation steps. This may include chipping, shredding, grinding, or drying to optimize feedstock characteristics such as particle size, moisture content, and chemical composition. Proper feedstock preparation ensures uniform feeding and efficient conversion in the gasification process.
- Gasification Reactor: The heart of the biomass gasification plant is the gasification reactor, where the biomass feedstock undergoes thermochemical conversion to produce syngas. Gasification reactors can take various forms, including fixed-bed, fluidized bed, entrained-flow, or hybrid configurations. These reactors operate at elevated temperatures (>700°C) and in the presence of a gasification agent (air, oxygen, steam) to promote the partial oxidation and decomposition of biomass into syngas constituents.
- Gas Cleaning and Conditioning: The raw syngas produced from the gasification reactor contains impurities such as tar, particulate matter, sulfur compounds, and trace metals, which must be removed or reduced to acceptable levels before further utilization. Gas cleaning and conditioning processes may include filtration, scrubbing, catalytic conversion, and cooling to improve syngas quality, stability, and compatibility with downstream applications.
- Syngas Utilization: Cleaned and conditioned syngas can be utilized in various downstream processes and applications, including:
- Power Generation: Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity and heat in combined heat and power (CHP) systems.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, synthetic diesel, and Fischer-Tropsch fuels through catalytic synthesis processes.
- Chemical Synthesis: Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols.
- Hydrogen Production: Syngas can be converted into pure hydrogen (H2) through processes such as water-gas shift reaction (WGSR) or steam methane reforming (SMR) for use in fuel cells, refining, and chemical synthesis.
- Residue Management: Residues generated during the gasification process, such as ash, char, and tar, are collected and managed to minimize environmental impacts and maximize resource recovery. Ash may be utilized as a soil amendment or construction material, while char can be recycled as a soil conditioner or carbon source. Tar and other liquid byproducts may undergo further processing or treatment for disposal or value-added utilization.
Biomass gasification plants offer several advantages over traditional combustion-based systems, including higher energy efficiency, lower emissions, and greater fuel flexibility. They enable the conversion of diverse biomass feedstocks into valuable energy products, contributing to renewable energy deployment, waste reduction, and sustainable development goals. Ongoing research and development efforts focus on advancing gasification technologies, optimizing process efficiency, and expanding the range of feedstocks and applications for biomass gasification plants.
Biomass Gasification Process
Biomass gasification is a thermochemical conversion process that converts biomass feedstocks into a gaseous mixture known as syngas (synthesis gas). This process involves the partial oxidation of biomass in a controlled environment to produce a combustible gas containing hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other trace gases. The syngas produced can be used for various energy applications, including power generation, heat production, biofuels synthesis, and chemical manufacturing.
Here’s a detailed overview of the biomass gasification process:
- Feedstock Preparation: Biomass feedstocks such as wood chips, agricultural residues, energy crops, or organic waste are first collected, sorted, and prepared for gasification. Feedstock preparation may involve shredding, chipping, drying, and sizing to optimize characteristics such as particle size, moisture content, and chemical composition. Proper feedstock preparation ensures efficient handling, feeding, and conversion in the gasification reactor.
- Gasification Reactor: The prepared biomass feedstock is fed into a gasification reactor, where it undergoes thermochemical conversion in the presence of a gasification agent. Gasification reactors can be classified into various types, including fixed-bed, fluidized bed, entrained-flow, and hybrid configurations. These reactors operate at elevated temperatures (>700°C) and in controlled atmospheres to promote the partial oxidation and decomposition of biomass into syngas constituents.
- Gasification Reactions: Within the gasification reactor, several thermochemical reactions occur sequentially, resulting in the production of syngas:
- Drying: Moisture present in the biomass feedstock is first evaporated and removed at temperatures below 100°C.
- Pyrolysis: The dried biomass undergoes thermal decomposition in the absence of oxygen to produce volatile gases, tar, and char. Pyrolysis occurs at temperatures typically ranging from 200°C to 700°C, depending on the biomass composition and heating rate.
- Char Gasification: The char residue from pyrolysis reacts with a gasification agent (air, oxygen, steam) at high temperatures (>700°C) to produce syngas constituents, primarily hydrogen (H2) and carbon monoxide (CO). The gasification reactions involve the partial oxidation of carbon (C) with oxygen (O2), water vapor (H2O), or carbon dioxide (CO2) to generate syngas and heat.
- Gas Cleaning and Conditioning: The raw syngas produced from the gasification reactor contains impurities such as tar, particulate matter, sulfur compounds, and trace metals, which must be removed or reduced to acceptable levels before further utilization. Gas cleaning and conditioning processes may include filtration, scrubbing, catalytic conversion, and cooling to improve syngas quality, stability, and compatibility with downstream applications.
- Syngas Utilization: Cleaned and conditioned syngas can be utilized in various downstream processes and applications, including:
- Power Generation: Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity and heat in combined heat and power (CHP) systems.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, synthetic diesel, and Fischer-Tropsch fuels through catalytic synthesis processes.
- Chemical Synthesis: Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols.
Biomass gasification offers several advantages over traditional combustion-based systems, including higher energy efficiency, lower emissions, and greater fuel flexibility. It enables the conversion of diverse biomass feedstocks into valuable energy products, contributing to renewable energy deployment, waste reduction, and sustainable development goals. Ongoing research and development efforts focus on advancing gasification technologies, optimizing process efficiency, and expanding the range of feedstocks and applications for biomass gasification.
Syngas Cleanup
Syngas cleanup is a crucial step in the biomass gasification process, aimed at removing impurities and contaminants from the raw syngas produced in the gasification reactor. The syngas generated from biomass gasification typically contains various undesired components, including tar, particulate matter, sulfur compounds, nitrogen oxides (NOx), trace metals, and ash particles. These impurities can have detrimental effects on downstream equipment, catalysts, and processes if not properly removed or reduced to acceptable levels. Syngas cleanup technologies are employed to enhance the quality, stability, and compatibility of syngas for subsequent utilization in power generation, biofuels production, chemical synthesis, and other applications.
Here are some key aspects of syngas cleanup:
- Tar Removal: Tar is a complex mixture of organic compounds formed during the pyrolysis and gasification of biomass feedstocks. Tar can cause fouling, corrosion, and catalyst deactivation in downstream equipment and processes. Various tar removal techniques are employed in syngas cleanup, including tar cracking, tar reforming, tar condensation, and tar scrubbing. These methods aim to decompose or remove tar compounds through thermal, catalytic, or physical processes to improve syngas quality and stability.
- Particulate Removal: Particulate matter, including char particles, ash, and unburned carbon residues, may be present in the raw syngas stream from biomass gasification. Particulate removal is essential to prevent fouling, erosion, and abrasion in downstream equipment such as gas turbines, engines, and heat exchangers. Filtration, cyclones, electrostatic precipitators, and scrubbers are commonly used to remove particulate matter from syngas streams through mechanical or electrostatic separation methods.
- Sulfur and Nitrogen Removal: Sulfur compounds (e.g., hydrogen sulfide, sulfur dioxide) and nitrogen oxides (NOx) can be present in syngas streams as impurities or byproducts of biomass gasification. These compounds are corrosive, toxic, and can inhibit catalysts in downstream processes. Syngas cleanup technologies such as desulfurization and denitrification processes are employed to remove sulfur and nitrogen compounds from syngas through chemical absorption, adsorption, or catalytic conversion reactions.
- Trace Metal Removal: Trace metals, such as mercury, arsenic, lead, and cadmium, may be present in biomass feedstocks or introduced during gasification processes. These metals can poison catalysts, promote corrosion, and pose environmental and health risks if emitted into the atmosphere. Syngas cleanup systems utilize sorbents, adsorbents, or catalytic converters to capture and remove trace metal contaminants from syngas streams through chemical or physical processes.
- Gas Cooling and Conditioning: Syngas cleanup processes may involve gas cooling and conditioning to adjust temperature, pressure, and moisture levels for downstream utilization or storage. Cooling syngas helps condense water vapor and other volatile compounds, facilitating their removal and improving syngas quality. Gas conditioning systems may include heat exchangers, condensers, and compressors to control syngas temperature, pressure, and humidity.
Syngas cleanup technologies play a critical role in maximizing the efficiency, reliability, and environmental performance of biomass gasification systems. By removing impurities and contaminants from syngas streams, these technologies enable the production of clean and high-quality syngas suitable for a wide range of energy and chemical applications. Ongoing research and development efforts focus on advancing syngas cleanup technologies, optimizing process performance, and reducing costs to enable the widespread deployment of biomass gasification for renewable energy production and sustainable development.
Syngas Utilization
Syngas, produced through biomass gasification, is a versatile energy carrier that can be utilized in various industrial processes and applications. Its composition, primarily consisting of hydrogen (H2) and carbon monoxide (CO), along with other gases such as carbon dioxide (CO2), methane (CH4), and trace impurities, makes it suitable for power generation, biofuels production, chemical synthesis, and other uses. Syngas utilization plays a pivotal role in the development of sustainable energy systems by harnessing renewable resources and reducing greenhouse gas emissions.
Here are some key applications of syngas utilization:
- Power Generation:Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity and heat in combined heat and power (CHP) systems. Gasification-based power plants utilize syngas as a fuel to drive electricity generators, providing a reliable and renewable source of energy for grid-connected and off-grid applications. The high energy content of syngas and the efficiency of modern power generation technologies contribute to the competitiveness of biomass gasification in the electricity market.
- Biofuels Production:Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, synthetic diesel, and Fischer-Tropsch fuels through catalytic synthesis processes. These biofuels offer cleaner alternatives to conventional fossil fuels, with reduced greenhouse gas emissions and lower environmental impact. Syngas-derived biofuels can be blended with petroleum-based fuels or used as standalone alternatives in transportation, heating, and industrial applications, contributing to energy security and sustainability objectives.
- Chemical Synthesis:Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols. These chemicals serve as building blocks for a wide range of downstream industries, including agriculture, pharmaceuticals, plastics, and manufacturing. Syngas-derived chemicals offer renewable alternatives to petrochemical-based products, reducing dependence on fossil fuels and mitigating environmental impacts associated with chemical production.
- Hydrogen Production:Syngas can be converted into pure hydrogen (H2) through processes such as water-gas shift reaction (WGSR) or steam methane reforming (SMR). Hydrogen is a clean and versatile energy carrier used in fuel cells, ammonia production, refining, and chemical synthesis. Syngas-derived hydrogen plays a crucial role in decarbonizing transportation, industry, and power sectors by enabling the transition to hydrogen-based energy systems and facilitating renewable integration.
- Carbon Capture and Utilization (CCU):Syngas can serve as a feedstock for carbon capture and utilization (CCU) processes, where carbon dioxide (CO2) is captured from syngas streams and converted into value-added products such as synthetic fuels, chemicals, and building materials. CCU technologies offer a pathway to mitigate greenhouse gas emissions by recycling CO2 into useful commodities, thereby closing the carbon cycle and promoting circular economy principles.
Syngas utilization represents a key strategy for maximizing the value and sustainability of biomass gasification systems. By converting renewable biomass feedstocks into clean energy products and industrial commodities, syngas enables the transition towards a low-carbon economy and contributes to global efforts to mitigate climate change and secure energy supplies. Continued innovation, investment, and deployment of syngas utilization technologies are essential for realizing the full potential of biomass gasification as a cornerstone of sustainable energy systems.
Gasification Biomass Feedstock
In the context of biomass gasification, the term “feedstock” refers to the organic material used as the primary input for the gasification process. The selection of an appropriate feedstock is critical to the efficiency, economics, and environmental sustainability of biomass gasification systems. A wide range of biomass feedstocks can be utilized for gasification, including:
- Wood and Forestry Residues:Wood biomass, including logs, chips, sawdust, bark, and forestry residues, is one of the most common feedstocks for biomass gasification. These materials are readily available from forestry operations, sawmills, and wood processing industries. Wood biomass offers advantages such as high energy density, low moisture content, and relatively uniform composition, making it suitable for gasification processes.
- Agricultural Residues:Agricultural residues, such as straw, husks, stalks, and bagasse, are abundant byproducts of crop cultivation and harvesting. These residues can be used as feedstocks for biomass gasification, providing a renewable source of energy while reducing agricultural waste and emissions. Agricultural residues vary in composition and availability depending on crop types, seasons, and geographic regions.
- Energy Crops:Energy crops are specifically cultivated for biomass production and energy generation purposes. These crops, including switchgrass, miscanthus, willow, and poplar, offer high biomass yields and rapid growth rates, making them suitable feedstocks for biomass gasification. Energy crops can be grown on marginal lands unsuitable for food crops, contributing to sustainable land use practices and biomass supply chains.
- Organic Waste:Organic waste materials, such as municipal solid waste (MSW), food waste, sewage sludge, and animal manure, can be utilized as feedstocks for biomass gasification. Recycling organic waste streams into energy products reduces landfilling, methane emissions, and environmental pollution while providing renewable energy and resource recovery opportunities. Organic waste feedstocks may require preprocessing and conditioning to remove contaminants and improve gasification performance.
- Algae and Aquatic Biomass:Algae and aquatic biomass, including microalgae, seaweed, and aquatic plants, are potential feedstocks for biomass gasification. These biomass sources offer high productivity, rapid growth rates, and minimal land requirements compared to terrestrial crops. Algae cultivation can utilize non-arable land, saline water, and wastewater resources, providing additional environmental benefits and ecosystem services.
- Biodegradable Byproducts:Biodegradable byproducts from industries such as pulp and paper, food processing, and bioethanol production can serve as feedstocks for biomass gasification. These byproducts, including black liquor, spent grains, and lignocellulosic residues, contain organic carbon suitable for energy recovery and value-added utilization. Gasification of biodegradable byproducts can improve resource efficiency, reduce waste disposal costs, and enhance overall process sustainability.
The selection of a biomass feedstock for gasification depends on factors such as feedstock availability, cost, quality, energy content, logistics, and environmental considerations. Feedstock characteristics such as moisture content, ash content, and chemical composition influence gasification performance, syngas quality, and downstream applications. Integrating diverse feedstocks into biomass gasification systems can enhance feedstock flexibility, supply chain resilience, and overall system sustainability. Ongoing research and development efforts focus on optimizing feedstock utilization, improving gasification technology, and advancing biomass-to-energy conversion pathways for renewable energy production.
Biomass Gasification Reactor
The biomass gasification reactor is a critical component of biomass gasification systems, where the thermochemical conversion of biomass feedstocks into syngas (synthesis gas) occurs. The design and operation of the gasification reactor significantly influence gasification performance, syngas composition, and overall system efficiency. Various reactor configurations and technologies are employed to achieve efficient biomass conversion while minimizing environmental impacts and maximizing syngas quality.
Here are the key aspects of biomass gasification reactors:
- Reactor Types:Biomass gasification reactors can be classified into several types based on their design, operating principles, and flow regimes. Common reactor configurations include:
- Fixed-Bed Gasifiers: Biomass feedstock is placed in a fixed bed within the reactor, and gasification reactions occur as a downward-moving gasification agent (air, oxygen, steam) flows through the bed. Fixed-bed gasifiers include updraft, downdraft, and cross-draft configurations, each offering unique advantages in terms of tar reduction, heat transfer, and syngas quality.
- Fluidized Bed Gasifiers: Biomass particles are suspended and fluidized by a gasification agent (typically air or steam) within the reactor, promoting intimate contact between the biomass and the gas phase. Fluidized bed gasifiers offer high heat and mass transfer rates, uniform temperature distribution, and enhanced tar cracking capabilities, making them suitable for a wide range of biomass feedstocks.
- Entrained-Flow Gasifiers: Biomass feedstock is entrained and rapidly converted into syngas as a high-velocity gasification agent (oxygen or steam) carries the biomass particles through the reactor. Entrained-flow gasifiers operate at high temperatures and pressures, enabling efficient biomass conversion, tar destruction, and syngas cleanup. They are commonly used in large-scale gasification plants for power generation and biofuels production.
- Operating Conditions:Gasification reactor operating conditions, including temperature, pressure, residence time, and gasification agent ratio, play a crucial role in determining gasification performance and syngas composition. Elevated temperatures (>700°C) are typically required to initiate and sustain gasification reactions, promoting the thermal decomposition of biomass into syngas constituents. Control of residence time and gasification agent flow rates ensures sufficient contact between the biomass and the gas phase, optimizing conversion efficiency and syngas yield.
- Gasification Mechanisms:Biomass gasification involves several thermochemical reactions, including pyrolysis, oxidation, reforming, and gasification. Pyrolysis converts biomass into volatile gases, tar, and char at elevated temperatures in the absence of oxygen. Oxidation reactions consume oxygen and produce heat, promoting the partial oxidation of carbon in the biomass to carbon monoxide (CO) and hydrogen (H2). Reforming reactions further convert tar and volatile hydrocarbons into CO and H2 through steam reforming, water-gas shift, and tar cracking mechanisms.
- Tar Reduction and Management:Tar compounds, formed during biomass pyrolysis and gasification, can condense and accumulate in gasification reactors, causing fouling, corrosion, and catalyst deactivation. Tar reduction and management strategies are employed to mitigate these issues and improve syngas quality. These strategies include reactor design modifications, operating parameter adjustments, tar cracking catalysts, and downstream tar removal systems such as scrubbers and filters.
- Syngas Quality and Cleanup:The design and operation of the gasification reactor significantly influence syngas composition, quality, and contaminants levels. Gasification reactor technologies that promote efficient biomass conversion and tar destruction contribute to cleaner syngas with reduced tar, particulate matter, sulfur compounds, and trace contaminants. Syngas cleanup systems further refine and condition the raw syngas to meet specific quality requirements for downstream applications, such as power generation, biofuels production, and chemical synthesis.
The selection of a biomass gasification reactor depends on factors such as feedstock characteristics, scale of operation, desired syngas quality, and process requirements. Advances in reactor design, materials, and control systems continue to improve gasification performance, flexibility, and reliability, driving the deployment of biomass gasification as a key technology for renewable energy production and sustainable development.
Gasification Process Efficiency
Gasification process efficiency refers to the effectiveness with which biomass feedstocks are converted into syngas (synthesis gas) while minimizing energy losses and environmental impacts. Improving gasification process efficiency is essential for maximizing the utilization of biomass resources, enhancing energy conversion performance, and achieving economic viability in biomass-to-energy systems. Several factors influence the efficiency of the gasification process:
- Feedstock Characteristics:The composition, moisture content, particle size, and chemical properties of biomass feedstocks significantly impact gasification process efficiency. High-quality feedstocks with low moisture content, uniform particle size distribution, and favorable chemical composition promote efficient biomass conversion and syngas production. Proper feedstock preparation and handling are essential to optimize gasification performance and minimize energy losses.
- Gasification Reactor Design:The design and configuration of the gasification reactor play a crucial role in determining gasification process efficiency. Factors such as reactor type (e.g., fixed-bed, fluidized bed, entrained-flow), operating parameters (e.g., temperature, pressure, residence time), and gasification agent selection influence biomass conversion kinetics, syngas yield, and tar formation. Advanced reactor designs and technologies that enhance heat and mass transfer, minimize heat losses, and promote uniform biomass conversion contribute to improved gasification efficiency.
- Gasification Agent:The choice of gasification agent (e.g., air, oxygen, steam) affects gasification process efficiency and syngas composition. Oxygen-blown gasification systems offer higher reaction rates and syngas yields compared to air-blown systems due to the absence of nitrogen dilution and reduced gas volume. Steam addition promotes steam reforming reactions, increases hydrogen yield, and reduces tar formation, leading to cleaner syngas production. Selecting the appropriate gasification agent based on process requirements and feedstock characteristics is essential for optimizing gasification efficiency.
- Operating Conditions:Gasification process efficiency is influenced by operating conditions such as temperature, pressure, gasification agent ratio, and residence time. Elevated temperatures (>700°C) promote biomass pyrolysis, char gasification, and tar cracking reactions, leading to higher syngas yields and cleaner gasification products. Control of residence time and gasification agent flow rates ensures sufficient contact between the biomass and the gas phase, optimizing conversion efficiency and energy utilization.
- Syngas Cleanup and Conditioning:Efficient syngas cleanup and conditioning systems are essential for improving gasification process efficiency and syngas quality. Tar removal, particulate filtration, sulfur and nitrogen removal, and trace metal capture contribute to cleaner syngas streams with reduced contaminants levels. Gas cooling and conditioning technologies adjust syngas temperature, pressure, and humidity for downstream utilization or storage, minimizing energy losses and enhancing syngas compatibility with end-use applications.
- Waste Heat Recovery:Waste heat recovery systems capture and utilize heat generated during the gasification process for various purposes, such as preheating biomass feedstocks, generating steam for process heating, or producing electricity through steam turbines or organic Rankine cycles. Efficient utilization of waste heat improves overall energy efficiency, reduces energy costs, and enhances the economic viability of biomass gasification systems.
Optimizing gasification process efficiency requires a holistic approach that integrates feedstock selection, reactor design, operating parameters, syngas cleanup, and waste heat utilization. Continuous research, development, and innovation efforts focus on advancing gasification technologies, enhancing system performance, and reducing environmental impacts to enable the widespread deployment of biomass gasification for renewable energy production and sustainable development.
Gasification Biomass to Energy Conversion
Gasification biomass to energy conversion refers to the process of converting biomass feedstocks into useful energy products, primarily in the form of syngas (synthesis gas), through thermochemical gasification reactions. This process involves the transformation of organic materials into a combustible gas mixture containing hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other trace gases. The syngas produced can be utilized for various energy applications, including power generation, heat production, biofuels synthesis, and chemical manufacturing. Here’s a detailed explanation of gasification biomass to energy conversion:
- Biomass Feedstock Preparation:The biomass feedstock undergoes preprocessing and preparation to optimize its characteristics for gasification. This may include drying to reduce moisture content, size reduction (chipping, shredding) to increase surface area and improve handling, and removal of contaminants and impurities. Proper feedstock preparation ensures efficient conversion and high-quality syngas production.
- Gasification Reactor Operation:The prepared biomass feedstock is fed into a gasification reactor, where it undergoes thermochemical conversion in the presence of a gasification agent (air, oxygen, steam). The gasification reactor operates at elevated temperatures (>700°C) and controlled conditions to promote the partial oxidation and decomposition of biomass into syngas constituents. Various reactor designs, such as fixed-bed, fluidized bed, and entrained-flow gasifiers, are employed to achieve efficient biomass conversion.
- Thermochemical Reactions:Within the gasification reactor, several thermochemical reactions occur sequentially, leading to the production of syngas:
- Drying and Pyrolysis: Moisture present in the biomass is first evaporated and removed, followed by thermal decomposition (pyrolysis) of the biomass into volatile gases, tar, and char in the absence of oxygen.
- Char Gasification: The char residue from pyrolysis reacts with the gasification agent (oxygen, steam) at high temperatures to produce syngas constituents, primarily hydrogen (H2) and carbon monoxide (CO), through partial oxidation and reforming reactions.
- Syngas Cleanup and Conditioning:The raw syngas produced from the gasification reactor contains impurities such as tar, particulate matter, sulfur compounds, and trace metals, which must be removed or reduced to acceptable levels before utilization. Syngas cleanup and conditioning systems, including filtration, scrubbing, catalytic conversion, and cooling, are employed to improve syngas quality, stability, and compatibility with downstream applications.
- Syngas Utilization:Cleaned and conditioned syngas can be utilized in various energy conversion processes and applications, including:
- Power Generation: Syngas can be combusted in gas turbines, reciprocating engines, or fuel cells to generate electricity and heat in combined heat and power (CHP) systems.
- Biofuels Production: Syngas serves as a feedstock for the production of biofuels such as ethanol, methanol, synthetic diesel, and Fischer-Tropsch fuels through catalytic synthesis processes.
- Chemical Synthesis: Syngas is a key precursor for the synthesis of various chemicals and industrial products, including ammonia, methanol, hydrogen, synthetic natural gas (SNG), and higher alcohols.
Gasification biomass to energy conversion offers several advantages over conventional combustion-based systems, including higher energy efficiency, lower emissions, and greater fuel flexibility. It enables the conversion of diverse biomass feedstocks into valuable energy products, contributing to renewable energy deployment, waste reduction, and sustainable development goals. Ongoing research and development efforts focus on advancing gasification technologies, optimizing process efficiency, and expanding the range of feedstocks and applications for biomass-to-energy conversion.
Biomass Gasification Economics:
Biomass gasification offers a promising avenue for renewable energy production, but its economic viability depends on various factors, including feedstock costs, capital investment, operating expenses, energy market prices, and government incentives. Understanding the economics of biomass gasification is crucial for evaluating project feasibility, estimating returns on investment, and informing decision-making processes. Here’s an overview of the key economic aspects of biomass gasification:
- Feedstock Costs:The cost of biomass feedstock constitutes a significant portion of the overall economics of biomass gasification. Feedstock costs depend on factors such as feedstock type, availability, transportation distance, seasonality, and market demand. Low-cost feedstocks, such as forestry residues, agricultural residues, and waste materials, enhance the economic competitiveness of biomass gasification projects.
- Capital Investment:The capital investment required for biomass gasification projects includes costs associated with plant construction, equipment procurement, site preparation, infrastructure development, and engineering and design services. Capital costs vary depending on project scale, technology selection, site-specific conditions, regulatory requirements, and project financing terms. Access to financing options, grants, subsidies, and incentives can mitigate upfront capital investment requirements and improve project economics.
- Operating Expenses:Operating expenses (OPEX) encompass the ongoing costs associated with biomass gasification plant operation, maintenance, labor, utilities, consumables, and feedstock procurement. OPEX also include costs related to syngas cleanup, conditioning, and product distribution. Optimizing operating expenses through efficient plant design, process integration, and maintenance practices is essential for maximizing project profitability and long-term viability.
- Energy Market Prices:Biomass gasification projects generate revenue by selling syngas, electricity, heat, biofuels, or chemical products to energy markets, industrial consumers, utilities, or end-users. Energy market prices for these products are influenced by factors such as commodity prices, supply and demand dynamics, regulatory policies, and market competition. Analyzing energy market prices and demand forecasts is essential for revenue projections and financial modeling of biomass gasification projects.
- Revenue Streams:Biomass gasification projects may generate revenue from multiple streams, including electricity sales, renewable energy credits (RECs), capacity payments, heat sales, biofuels production, chemical sales, carbon credits, and byproduct utilization. Diversifying revenue streams can enhance project resilience to market fluctuations and regulatory changes, mitigate financial risks, and improve overall project economics.
- Financial Metrics:Various financial metrics are used to assess the economic performance and viability of biomass gasification projects, including net present value (NPV), internal rate of return (IRR), payback period, levelized cost of energy (LCOE), return on investment (ROI), and cash flow analysis. These metrics evaluate project economics, profitability, risk-adjusted returns, and sensitivity to key parameters such as feedstock costs, energy prices, and discount rates.
- Policy and Regulatory Support:Government policies, incentives, subsidies, and regulatory frameworks play a crucial role in shaping the economic landscape for biomass gasification projects. Supportive policies, such as renewable energy mandates, tax credits, feed-in tariffs, and grants, can significantly improve the financial attractiveness and competitiveness of biomass gasification investments. Understanding and leveraging policy incentives are essential for maximizing project economics and attracting investment.
Overall, the economics of biomass gasification projects depend on a complex interplay of feedstock availability, technology costs, energy market dynamics, policy support, and project-specific factors. Conducting comprehensive techno-economic assessments, risk analyses, and financial modeling is essential for evaluating project feasibility, identifying investment opportunities, and optimizing the economic performance of biomass gasification ventures.
Biomass Gasification Environmental Impacts:
Biomass gasification offers a renewable and potentially sustainable pathway for energy production; however, it also has environmental implications that need careful consideration. Understanding and mitigating these impacts are crucial for ensuring that biomass gasification contributes positively to overall environmental goals. Here are the key environmental impacts associated with biomass gasification:
- Greenhouse Gas Emissions:Biomass gasification is often considered carbon-neutral because the carbon dioxide (CO2) emitted during gasification is offset by the carbon absorbed by the biomass during its growth. However, incomplete combustion or inefficient gasification processes can result in emissions of greenhouse gases such as methane (CH4) and nitrous oxide (N2O), which have higher global warming potentials than CO2. Minimizing emissions through proper gasification technology selection, operation, and emissions control measures is essential for mitigating climate impacts.
- Air Quality:Biomass gasification can emit air pollutants such as particulate matter (PM), volatile organic compounds (VOCs), nitrogen oxides (NOx), sulfur dioxide (SO2), and carbon monoxide (CO), especially during startup, shutdown, or under suboptimal operating conditions. These pollutants can contribute to local air quality degradation, respiratory health issues, and environmental pollution. Implementing effective emission control technologies, such as electrostatic precipitators, scrubbers, and catalytic converters, can reduce air pollutant emissions from gasification plants.
- Land Use and Biodiversity:Biomass feedstock production for gasification may compete with land use for food crops, natural habitats, and ecosystem services, leading to land conversion, habitat loss, and biodiversity impacts. Sustainable land management practices, including agroforestry, crop rotation, and land restoration, can minimize negative land use impacts and promote ecosystem resilience. Utilizing waste biomass and marginal lands for feedstock production can reduce pressure on agricultural land and mitigate land use conflicts.
- Water Resources:Biomass gasification requires water for feedstock preparation, reactor cooling, syngas cleanup, and wastewater treatment. Water consumption and discharge from gasification plants can affect local water resources, aquatic ecosystems, and water quality. Implementing water conservation measures, recycling wastewater streams, and minimizing chemical usage can reduce the water footprint and environmental impact of biomass gasification operations.
- Solid Waste Management:Biomass gasification produces solid residues, such as ash, char, and tar, which require proper management to prevent environmental contamination and ecosystem disruption. Ash disposal, tar handling, and byproduct utilization should be managed to minimize land, air, and water pollution risks. Recycling ash for soil amendment, utilizing char for carbon sequestration, and converting tar into value-added products can enhance resource efficiency and reduce waste generation.
- Ecological Footprint:Biomass feedstock production, harvesting, transportation, and processing activities contribute to the ecological footprint associated with biomass gasification. Assessing and minimizing the ecological impacts of these activities, such as soil erosion, habitat fragmentation, and invasive species introduction, are essential for ensuring the sustainability of biomass supply chains. Adopting sustainable forestry practices, promoting biodiversity conservation, and enhancing ecosystem services can mitigate ecological footprint impacts.
Addressing the environmental impacts of biomass gasification requires a holistic approach that integrates technology innovation, policy support, stakeholder engagement, and best management practices. Implementing environmental management systems, conducting life cycle assessments, and adhering to regulatory requirements are essential for ensuring the sustainable development of biomass gasification projects. By mitigating environmental risks and maximizing environmental co-benefits, biomass gasification can contribute to a more sustainable and resilient energy future.
EMS Power Machines
We design, manufacture and assembly Power Machines such as – diesel generators, electric motors, vibration motors, pumps, steam engines and steam turbines
EMS Power Machines is a global power engineering company, one of the five world leaders in the industry in terms of installed equipment. The companies included in the company have been operating in the energy market for more than 60 years.
EMS Power Machines manufactures steam turbines, gas turbines, hydroelectric turbines, generators, and other power equipment for thermal, nuclear, and hydroelectric power plants, as well as for various industries, transport, and marine energy.
EMS Power Machines is a major player in the global power industry, and its equipment is used in power plants all over the world. The company has a strong track record of innovation, and it is constantly developing new and improved technologies.
Here are some examples of Power Machines’ products and services:
- Steam turbines for thermal and nuclear power plants
- Gas turbines for combined cycle power plants and industrial applications
- Hydroelectric turbines for hydroelectric power plants
- Generators for all types of power plants
- Boilers for thermal power plants
- Condensers for thermal power plants
- Reheaters for thermal power plants
- Air preheaters for thermal power plants
- Feedwater pumps for thermal power plants
- Control systems for power plants
- Maintenance and repair services for power plants
EMS Power Machines is committed to providing its customers with high-quality products and services. The company has a strong reputation for reliability and innovation. Power Machines is a leading provider of power equipment and services, and it plays a vital role in the global power industry.
EMS Power Machines, which began in 1961 as a small factory of electric motors, has become a leading global supplier of electronic products for different segments. The search for excellence has resulted in the diversification of the business, adding to the electric motors products which provide from power generation to more efficient means of use.
