Pyrolysis is a thermal decomposition process that involves the degradation of organic materials in the absence of oxygen or with limited oxygen supply, typically at elevated temperatures ranging from 300°C to 900°C. This thermochemical process breaks down complex organic compounds into simpler molecules, producing a range of valuable products such as biochar, bio-oil, and syngas. Pyrolysis is a key technology in the field of biomass conversion, waste management, and renewable energy production.
Pyrolysis
During pyrolysis, the organic material undergoes several simultaneous reactions, including dehydration, depolymerization, and cracking. These reactions result in the breakdown of larger molecules present in the feedstock into smaller molecules, without complete combustion. The absence of oxygen prevents the formation of combustion products such as carbon dioxide and water vapor, which distinguishes pyrolysis from combustion and gasification processes.
The products obtained from pyrolysis depend on various factors such as the type of feedstock, pyrolysis temperature, heating rate, residence time, and reactor design. Generally, pyrolysis produces three main fractions:
- Biochar: Also known as charcoal, biochar is a carbon-rich solid residue produced during pyrolysis. Biochar is structurally stable and rich in carbon, making it a valuable soil amendment for improving soil fertility, water retention, and carbon sequestration. Additionally, biochar can be used as a renewable energy source or as a precursor for activated carbon production.
- Bio-oil: Bio-oil, also called pyrolysis oil or biocrude, is a dark, viscous liquid fraction obtained from pyrolysis. Bio-oil contains a complex mixture of oxygenated hydrocarbons, including phenols, ketones, aldehydes, and organic acids. Bio-oil has potential applications as a renewable fuel for heat and power generation, as well as a feedstock for producing biofuels, biochemicals, and specialty chemicals.
- Syngas: Syngas, short for synthesis gas, is a gaseous mixture comprising hydrogen, carbon monoxide, carbon dioxide, methane, and trace gases produced during pyrolysis. Syngas is a versatile intermediate that can be used for various purposes, including combustion for heat and power generation, synthesis of fuels and chemicals via catalytic processes, and as a reducing agent in metallurgical and chemical processes.
Pyrolysis technology offers several advantages, including the ability to convert a wide range of feedstocks, including biomass, waste plastics, and rubber, into valuable products. Additionally, pyrolysis processes can be integrated with existing industries for waste management, energy recovery, and resource utilization. However, challenges such as reactor design optimization, product quality control, and economic viability remain areas of active research and development in the field of pyrolysis.
Biomass:
Biomass refers to organic materials derived from plants, animals, and microorganisms that can be used as a renewable and sustainable source of energy. It encompasses a diverse range of feedstocks, including agricultural residues, forestry residues, energy crops, organic waste, and algae. Biomass can be converted into various forms of energy, such as heat, electricity, biofuels, and biogas, through different conversion technologies, including combustion, gasification, pyrolysis, and biochemical processes.
One of the key advantages of biomass is its abundant availability and renewable nature. Unlike fossil fuels, which are finite and non-renewable, biomass resources can be replenished through natural processes such as photosynthesis. This makes biomass an attractive option for reducing dependence on fossil fuels and mitigating greenhouse gas emissions associated with conventional energy sources.
Biomass can be utilized directly as a fuel for heating and cooking or processed into more advanced forms of energy through thermochemical or biochemical conversion pathways. Thermochemical conversion technologies, such as combustion, gasification, and pyrolysis, involve the application of heat to biomass to produce heat, electricity, biofuels, or chemicals. Biochemical conversion technologies, such as anaerobic digestion and fermentation, employ biological processes to convert biomass into biogas, ethanol, biodiesel, or other bio-based products.
In addition to its energy potential, biomass offers several environmental benefits, including carbon neutrality, as the carbon dioxide emitted during biomass combustion or conversion is offset by the carbon dioxide absorbed during plant growth. Biomass energy production also contributes to waste management by diverting organic waste from landfills and reducing methane emissions from decomposing organic matter.
However, the widespread adoption of biomass for energy purposes also poses challenges, including resource availability, logistics, and sustainability. Sustainable biomass production practices, such as agroforestry, crop rotation, and waste-to-energy systems, are essential to ensure the long-term viability of biomass as a renewable energy source. Moreover, advances in biomass conversion technologies and process optimization are needed to improve efficiency, reduce costs, and enhance the environmental performance of biomass energy systems.
Overall, biomass represents a valuable and versatile renewable energy resource that can play a significant role in the transition to a low-carbon and sustainable energy future. Continued research, investment, and policy support are essential to unlock the full potential of biomass and accelerate its deployment as a clean and renewable energy source.
Feedstock:
Feedstock refers to the raw material or substance used as input for a manufacturing process or industrial operation, particularly in the context of biomass conversion technologies. In the field of renewable energy and bio-based products, feedstock typically refers to organic materials derived from plants, animals, or microorganisms that can be converted into energy, fuels, chemicals, or other value-added products through various conversion processes.
Feedstocks for biomass conversion can include a wide range of materials, such as agricultural residues (e.g., crop residues, straw, husks), forestry residues (e.g., wood chips, sawdust, bark), energy crops (e.g., switchgrass, miscanthus, willow), organic waste (e.g., municipal solid waste, sewage sludge, food waste), algae, and dedicated energy crops grown specifically for energy production.
The choice of feedstock depends on several factors, including availability, cost, energy content, moisture content, composition, and suitability for specific conversion technologies. For example, lignocellulosic feedstocks such as wood, agricultural residues, and energy crops are well-suited for thermochemical conversion processes like combustion, gasification, and pyrolysis, which require materials with high cellulose, hemicellulose, and lignin content.
Feedstock quality and characteristics also play a crucial role in determining the efficiency and performance of biomass conversion processes. Factors such as moisture content, particle size, ash content, and chemical composition can affect process economics, energy yields, and environmental performance. Therefore, feedstock handling, preprocessing, and conditioning are often necessary to optimize feedstock properties and prepare them for conversion.
In addition to traditional biomass feedstocks, emerging feedstock sources such as algae and waste-derived feedstocks are gaining attention for their potential to expand feedstock availability, reduce competition with food production, and enhance sustainability. Algae, for example, offer high growth rates, high lipid content, and the ability to grow in non-arable land or wastewater, making them a promising feedstock for biofuel production.
Overall, feedstock selection plays a critical role in the development and deployment of biomass conversion technologies. Sustainable feedstock supply chains, efficient conversion processes, and integrated biorefinery concepts are essential for maximizing the value and sustainability of biomass feedstocks in the transition to a bio-based economy and a more sustainable energy future.
Thermal Decomposition:
Thermal decomposition, also known as thermolysis or pyrolysis, is a chemical process in which a compound is broken down into simpler substances or elements by the application of heat. This process occurs in the absence of oxygen or with limited oxygen supply, preventing complete combustion and allowing for the decomposition of the compound without the formation of combustion products such as carbon dioxide and water vapor.
The thermal decomposition process involves the breaking of chemical bonds within the compound, leading to the formation of smaller molecules, gases, and solid residues. The temperature at which thermal decomposition occurs varies depending on the nature of the compound and its chemical structure. Generally, higher temperatures accelerate the decomposition process by providing the necessary activation energy to break the bonds.
Thermal decomposition is widely used in various industrial processes, including the production of chemicals, fuels, and materials. In the context of biomass conversion, thermal decomposition plays a crucial role in processes such as pyrolysis, gasification, and combustion, where biomass feedstocks are thermally converted into biofuels, syngas, biochar, and heat.
During biomass pyrolysis, for example, thermal decomposition occurs at temperatures typically ranging from 300°C to 900°C, resulting in the breakdown of complex organic molecules present in the biomass feedstock into smaller molecules such as bio-oil, syngas, and biochar. Similarly, in biomass gasification, thermal decomposition of biomass occurs at elevated temperatures in the presence of a gasifying agent (e.g., steam, air, oxygen), leading to the production of syngas containing hydrogen, carbon monoxide, and other gases.
The kinetics of thermal decomposition are influenced by several factors, including temperature, heating rate, residence time, pressure, and the chemical composition of the feedstock. Understanding the thermodynamics and kinetics of thermal decomposition is essential for optimizing process parameters, maximizing product yields, and controlling the composition and quality of the final products.
Overall, thermal decomposition is a fundamental process in biomass conversion and other industrial applications, offering a versatile and efficient means of converting organic materials into valuable products while minimizing waste and environmental impact. Ongoing research and development efforts continue to advance our understanding of thermal decomposition processes and their potential applications in sustainable energy production and resource utilization.
Biochar:
Biochar is a carbon-rich solid material produced through the process of pyrolysis, which involves heating biomass in the absence of oxygen. It is a stable form of charcoal that is primarily composed of carbon, with small amounts of ash and other inorganic compounds. Biochar is known for its high porosity, large surface area, and capacity to improve soil fertility and carbon sequestration.
The production of biochar involves subjecting biomass feedstocks such as agricultural residues, forestry waste, or organic by-products to high temperatures (typically between 300°C and 900°C) in a low-oxygen or oxygen-free environment. During pyrolysis, volatile organic compounds and gases are released from the biomass, leaving behind a carbon-rich residue known as biochar.
Biochar has several beneficial properties that make it a valuable soil amendment and carbon sequestration tool. When incorporated into soil, biochar can improve soil structure, water retention, nutrient retention, and microbial activity. Its high porosity and surface area provide habitat and refuge for soil microorganisms, promoting soil health and fertility.
Furthermore, biochar has the ability to sequester carbon in the soil for long periods, thereby mitigating greenhouse gas emissions and contributing to climate change mitigation efforts. The stable carbon structure of biochar makes it resistant to microbial degradation, allowing it to persist in the soil for hundreds or even thousands of years.
In addition to its soil enhancement properties, biochar has potential applications in environmental remediation, wastewater treatment, livestock farming, and energy production. It can be used as a filtration medium to remove contaminants from water and air, as a feed additive to improve animal health and reduce methane emissions from livestock, and as a renewable fuel source for heat and power generation.
The production and application of biochar offer significant opportunities for sustainable agriculture, carbon sequestration, and waste management. However, challenges remain in terms of scalability, cost-effectiveness, and standardization of production methods. Ongoing research and development efforts are focused on optimizing biochar production processes, identifying suitable feedstocks, and assessing its long-term impacts on soil health, crop productivity, and carbon sequestration.
Thermochemical Conversion:
Thermochemical conversion is a process that involves the transformation of biomass or other organic materials into energy, fuels, and chemicals through the application of heat and chemical reactions. This conversion pathway relies on thermochemical reactions to break down complex organic compounds present in biomass feedstocks and produce useful energy products such as heat, electricity, biofuels, and biochemicals.
Thermochemical conversion technologies can be broadly classified into three main categories: combustion, gasification, and pyrolysis. Each of these processes utilizes different thermochemical reactions and operating conditions to convert biomass into energy products with varying compositions and properties.
- Combustion: Combustion is the most straightforward thermochemical conversion process, in which biomass is burned in the presence of oxygen to release heat energy. The heat generated from biomass combustion can be used directly for heating, drying, or power generation in boilers, furnaces, and power plants. Combustion systems vary in scale from small-scale residential stoves to large-scale industrial boilers and power plants. While combustion is a relatively simple and well-established technology, it can result in emissions of air pollutants such as particulate matter, nitrogen oxides, and sulfur dioxide if not properly controlled.
- Gasification: Gasification involves the partial oxidation of biomass at elevated temperatures (typically between 700°C and 1,200°C) in a low-oxygen or oxygen-starved environment to produce a mixture of gases known as syngas (synthesis gas). Syngas is composed primarily of hydrogen (H2) and carbon monoxide (CO), along with smaller amounts of methane (CH4) and other trace gases. Gasification offers several advantages over combustion, including higher energy efficiency, lower emissions, and greater flexibility in feedstock selection. Syngas produced from biomass gasification can be used as a fuel for internal combustion engines, gas turbines, fuel cells, or as a precursor for the production of biofuels and chemicals.
- Pyrolysis: Pyrolysis is a thermal decomposition process that occurs in the absence of oxygen or with limited oxygen supply, resulting in the production of bio-oil, biochar, and syngas. Pyrolysis typically takes place at temperatures ranging from 300°C to 800°C, depending on the desired product composition and properties. Fast pyrolysis involves rapid heating of biomass feedstocks to high temperatures (usually above 400°C) in a matter of seconds to produce bio-oil, while slow pyrolysis involves slower heating rates and longer residence times, favoring the production of biochar. Pyrolysis offers the advantage of producing a liquid bio-oil that can be upgraded into transportation fuels or used as a feedstock for biochemical processes.
Thermochemical conversion technologies play a vital role in the utilization of biomass as a renewable and sustainable energy resource. These processes offer a versatile and efficient means of converting biomass into a range of energy products while minimizing waste and environmental impact. Continued research and development efforts are focused on improving the efficiency, economics, and environmental performance of thermochemical conversion technologies to enable their widespread deployment in the transition to a low-carbon energy future.
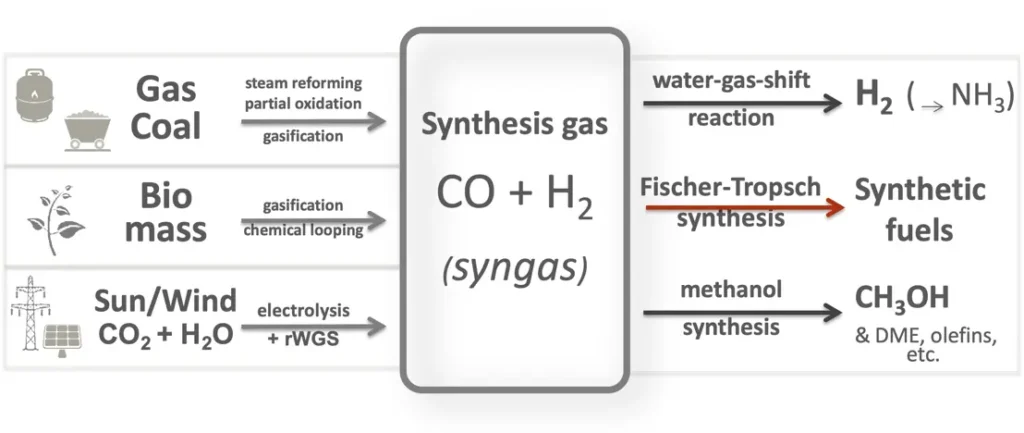
Syngas:
Syngas, short for synthesis gas, is a mixture of gases primarily composed of hydrogen (H2) and carbon monoxide (CO), along with lesser amounts of carbon dioxide (CO2), methane (CH4), and other trace gases. It is produced through thermochemical processes such as biomass gasification, coal gasification, steam reforming of natural gas, or partial oxidation of hydrocarbons.
The production of syngas typically involves the partial oxidation or gasification of carbon-containing feedstocks such as biomass, coal, or natural gas at elevated temperatures (>700°C) in the presence of a controlled amount of oxygen (O2), steam (H2O), or a combination of both. The composition of syngas can be adjusted by varying the operating conditions, feedstock composition, and gasification technology used.
Syngas is a versatile intermediate product that can be used as a fuel for power generation, heating, and as a feedstock for the production of a wide range of chemicals and fuels through further processing. Its composition makes it suitable for use in gas turbines, internal combustion engines, fuel cells, and chemical synthesis processes.
Applications of syngas include:
- Power Generation: Syngas can be burned directly in gas turbines or internal combustion engines to generate electricity. Combined cycle power plants that use syngas in gas turbines followed by steam turbines can achieve high efficiencies and flexibility in power generation.
- Heat Generation: Syngas can be used as a fuel for heating applications in industrial processes, district heating systems, and residential heating systems. It can replace natural gas or other fossil fuels in boilers, furnaces, and cogeneration systems to provide heat and hot water.
- Chemical Synthesis: Syngas serves as a precursor for the production of various chemicals and fuels through catalytic processes such as Fischer-Tropsch synthesis, methanol synthesis, ammonia synthesis, and hydrocarbon synthesis. These processes enable the production of synthetic fuels, hydrogen, methanol, ammonia, and other value-added chemicals from renewable or fossil feedstocks.
- Biofuels Production: Syngas derived from biomass gasification can be converted into liquid biofuels such as ethanol, dimethyl ether (DME), synthetic diesel, and aviation fuels through thermochemical or biochemical conversion processes. These biofuels offer renewable alternatives to conventional fossil fuels and can help reduce greenhouse gas emissions in the transportation sector.
Syngas production and utilization play a crucial role in the transition to a low-carbon economy by enabling the efficient conversion of biomass, coal, and other carbonaceous feedstocks into clean energy and valuable products. Continued research and development efforts focus on improving syngas production technologies, optimizing process efficiencies, and expanding the range of syngas-based applications to support sustainable energy systems and mitigate climate change.
Biomass Gasification:
Biomass gasification is a thermochemical process that converts biomass feedstocks into a combustible gas mixture called syngas (synthesis gas) in a controlled environment. This process involves heating biomass in the presence of a gasifying agent, typically steam, oxygen, or a combination of both, at high temperatures (>700°C) in a low-oxygen or oxygen-starved environment.
The biomass feedstock, which can include wood chips, agricultural residues, forestry waste, energy crops, or municipal solid waste, undergoes several chemical reactions during gasification:
- Drying: At the beginning of the process, moisture present in the biomass is removed through evaporation. This step is essential to prevent energy loss and ensure efficient gasification.
- Pyrolysis: As the temperature increases, the biomass undergoes thermal decomposition or pyrolysis, breaking down complex organic compounds into volatile gases, tars, and solid char. This step releases organic vapors and gases such as methane, ethylene, and other hydrocarbons, as well as tars and organic acids.
- Gasification: In the presence of the gasifying agent (steam or oxygen), the remaining char undergoes further reactions, primarily gasification and partial oxidation. These reactions convert the carbonaceous material into a mixture of gases consisting mainly of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and methane (CH4), along with trace amounts of other gases.
- Tar Cracking and Cleanup: The raw syngas produced during gasification contains impurities such as tars, particulates, and contaminants, which need to be removed to meet product specifications and prevent equipment fouling. Tar cracking and cleanup processes, such as filtration, scrubbing, and catalytic reforming, are employed to purify the syngas before further utilization.
Biomass gasification offers several advantages over other thermochemical conversion processes, including:
- Fuel Flexibility: Biomass gasification can utilize a wide range of feedstocks, including agricultural residues, forestry waste, energy crops, and organic by-products, making it a versatile technology for bioenergy production.
- Syngas Quality: The syngas produced through biomass gasification has a relatively high hydrogen-to-carbon ratio, making it suitable for use in a variety of applications, including power generation, heat production, and chemical synthesis.
- Energy Efficiency: Gasification processes can achieve high energy conversion efficiencies compared to other biomass conversion technologies, especially when combined with combined heat and power (CHP) systems or integrated with downstream processes for syngas upgrading and utilization.
- Carbon Sequestration: Biomass gasification coupled with carbon capture and storage (CCS) technologies can enable carbon-negative energy production, where carbon dioxide captured during gasification is permanently sequestered underground, effectively reducing net greenhouse gas emissions.
Biomass gasification has applications in various sectors, including power generation, industrial heating, transportation fuels, and chemical production. Continued research and development efforts focus on improving gasification technologies, optimizing process efficiencies, and expanding the range of feedstocks and applications to support the transition to a sustainable and low-carbon energy future.
Syngas Cleanup:
Syngas cleanup is a critical step in the biomass gasification process that involves removing impurities and contaminants from the raw syngas to meet quality specifications for downstream utilization. Raw syngas produced during gasification contains a range of impurities, including tars, particulates, sulfur compounds, ammonia, alkali metals, and trace contaminants, which can negatively impact the performance and efficiency of syngas utilization systems such as engines, turbines, fuel cells, and chemical reactors.
Syngas cleanup technologies are designed to achieve several objectives:
- Tar Removal: Tar compounds, which are complex organic molecules produced during biomass pyrolysis and gasification, can condense and form deposits on equipment surfaces, leading to fouling and corrosion. Tar removal technologies, such as filtration, scrubbing, and catalytic conversion, are employed to reduce tar concentrations in the syngas to acceptable levels.
- Particulate Removal: Particulate matter, including ash, char, and soot, can be present in the raw syngas stream, particularly when using solid biomass feedstocks. Particulate removal technologies, such as cyclones, filters, and electrostatic precipitators, are used to capture and remove solid particles from the syngas to prevent equipment fouling and protect downstream components.
- Sulfur Removal: Sulfur compounds, such as hydrogen sulfide (H2S) and carbonyl sulfide (COS), can be present in the raw syngas due to sulfur-containing compounds in the biomass feedstock or sulfur impurities in the gasifying agent. Sulfur removal technologies, including desulfurization sorbents, chemical scrubbers, and catalytic reactors, are employed to remove sulfur from the syngas to prevent corrosion, catalyst poisoning, and environmental emissions.
- Ammonia Removal: Ammonia (NH3) can be present in the raw syngas as a result of nitrogen-containing compounds in the biomass feedstock or from ammonia-based gasifying agents. Ammonia removal technologies, such as selective catalytic reduction (SCR) and ammonia scrubbing, are used to reduce ammonia concentrations in the syngas to prevent catalyst deactivation and environmental emissions.
- Alkali Metal Removal: Alkali metals, such as potassium (K) and sodium (Na), can be present in the raw syngas as a result of biomass composition or from alkali-based catalysts used in gasification processes. Alkali metal removal technologies, including sorbents, membranes, and chemical treatments, are employed to reduce alkali metal concentrations in the syngas to prevent corrosion, fouling, and catalyst deactivation.
Syngas cleanup technologies vary depending on the specific impurities present in the raw syngas, the desired syngas quality requirements, and the intended end-use applications. Integration of syngas cleanup systems with gasification processes is essential to ensure reliable and efficient syngas production for a wide range of energy and chemical applications. Continued research and development efforts focus on improving syngas cleanup technologies, optimizing process efficiency, and reducing costs to enable the widespread deployment of biomass gasification for clean and sustainable energy production.
Gasification Reactor:
The gasification reactor is the core component of a gasification system where biomass feedstocks are converted into syngas through thermochemical reactions in a controlled environment. The design and operation of the gasification reactor significantly influence the efficiency, performance, and product quality of the gasification process.
Gasification reactors can be classified based on their configuration, operating conditions, and reaction mechanisms. Common types of gasification reactors include:
- Fixed-Bed Gasifiers: Fixed-bed gasifiers consist of a stationary bed of biomass feedstock through which a gasifying agent, such as air, oxygen, or steam, is passed from the bottom to the top. The biomass undergoes pyrolysis, gasification, and combustion reactions as it moves through the reactor, resulting in the production of syngas. Fixed-bed gasifiers are relatively simple in design, but they may suffer from limitations related to heat transfer, bed agglomeration, and tar formation.
- Fluidized-Bed Gasifiers: Fluidized-bed gasifiers use a bed of inert material, such as sand or alumina, as a medium to suspend and fluidize the biomass feedstock. The gasifying agent is introduced into the bed, causing the biomass particles to become fluidized and mix intimately with the gas phase. Fluidized-bed gasifiers offer excellent heat and mass transfer characteristics, uniform temperature distribution, and enhanced tar cracking and cleanup capabilities compared to fixed-bed gasifiers.
- Entrained-Flow Gasifiers: Entrained-flow gasifiers entrain finely ground biomass particles into a high-velocity stream of gasifying agent, typically oxygen or steam, in a refractory-lined reactor. The biomass particles are rapidly heated and gasified as they are carried through the reactor, resulting in high syngas production rates and efficient tar cracking. Entrained-flow gasifiers operate at high temperatures and pressures, making them suitable for large-scale, high-throughput applications such as power generation and syngas production for chemical synthesis.
- Bubbling-Fluidized-Bed Gasifiers: Bubbling-fluidized-bed gasifiers utilize a fluidized bed of biomass particles in which gas bubbles are formed and rise through the bed. Biomass feedstock is continuously fed into the bed, where it undergoes gasification and partial oxidation reactions. Bubbling-fluidized-bed gasifiers offer good mixing and residence time characteristics, allowing for efficient gas-solid contact and enhanced tar conversion.
Gasification reactors are designed to achieve optimal conditions for biomass conversion while minimizing energy losses, tar formation, and reactor fouling. Key design considerations for gasification reactors include reactor geometry, residence time, temperature profile, gasification kinetics, heat transfer mechanisms, and process control strategies. Advances in reactor design, materials science, computational modeling, and process optimization continue to drive improvements in gasification technology, enabling the efficient and sustainable conversion of biomass into syngas for clean energy production and chemical synthesis applications.
Syngas Utilization:
Syngas utilization refers to the conversion and application of syngas (synthesis gas) produced through biomass gasification for various energy and chemical processes. Syngas, which primarily consists of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and methane (CH4), along with trace amounts of other gases, can be utilized in a wide range of applications, including power generation, heat production, transportation fuels, and chemical synthesis.
- Power Generation: Syngas can be used as a fuel for combustion in gas turbines, reciprocating engines, and combined heat and power (CHP) systems to generate electricity and heat. Gasification-based power generation offers high efficiency, low emissions, and fuel flexibility, making it suitable for decentralized and distributed energy systems.
- Heat Production: Syngas can be combusted directly in boilers, furnaces, and industrial heaters to produce steam or hot water for space heating, industrial processes, and district heating systems. The heat energy derived from syngas combustion can be used in various industrial applications, including drying, sterilization, and chemical processing.
- Transportation Fuels: Syngas can be converted into liquid transportation fuels such as methanol, ethanol, synthetic diesel, and synthetic gasoline through catalytic processes such as Fischer-Tropsch synthesis and methanol synthesis. These synthetic fuels offer energy density, compatibility with existing infrastructure, and reduced greenhouse gas emissions compared to conventional petroleum-based fuels.
- Chemical Synthesis: Syngas serves as a versatile feedstock for the production of a wide range of chemicals and intermediates, including methanol, ammonia, hydrogen, synthetic natural gas (SNG), olefins, and higher alcohols. These chemicals are used in various industries, including petrochemicals, fertilizers, plastics, pharmaceuticals, and specialty chemicals.
- Hydrogen Production: Syngas can be further processed via water-gas shift (WGS) reaction or membrane separation to produce high-purity hydrogen gas for fuel cells, hydrogenation reactions, and industrial processes. Hydrogen derived from syngas offers a clean and sustainable alternative to fossil fuel-based hydrogen production methods.
- Carbon Capture and Utilization (CCU): Syngas can be utilized in CCU processes to capture and convert carbon dioxide (CO2) emissions into value-added products such as chemicals, fuels, and building materials. CCU technologies enable the utilization of CO2 as a feedstock rather than a waste product, contributing to greenhouse gas mitigation and carbon management efforts.
Syngas utilization technologies continue to evolve with advancements in catalyst development, process engineering, and system integration, enabling the efficient and sustainable conversion of biomass-derived syngas into valuable energy and chemical products. Integrated approaches that combine gasification, syngas cleanup, and downstream conversion processes are essential to maximize the economic and environmental benefits of syngas utilization in the transition towards a low-carbon and circular economy.
Biomass Gasification Plant:
A biomass gasification plant is a facility designed to convert biomass feedstocks into syngas through the process of gasification. It consists of several integrated units and equipment, each serving a specific function in the biomass-to-energy conversion process. The plant layout and configuration may vary depending on factors such as feedstock type, scale of operation, and end-use applications. Here’s an overview of the key components typically found in a biomass gasification plant:
- Feedstock Handling System: This system is responsible for receiving, storing, and preparing biomass feedstocks for gasification. It may include equipment such as shredders, chippers, conveyors, and storage silos to handle various types of biomass materials, including wood chips, agricultural residues, energy crops, and organic wastes.
- Gasification Reactor: The gasification reactor is the heart of the plant where biomass feedstocks undergo thermochemical reactions to produce syngas. Different types of gasification reactors, such as fixed-bed, fluidized-bed, and entrained-flow reactors, may be used depending on factors such as feedstock characteristics, gasification technology, and process requirements.
- Syngas Cleanup System: The syngas cleanup system is responsible for removing impurities and contaminants from the raw syngas to meet quality specifications for downstream utilization. It typically includes components such as tar removal units, particulate filters, desulfurization units, ammonia scrubbers, and alkali metal removal systems.
- Gas Cooling and Conditioning: After cleanup, the hot syngas is cooled and conditioned to reduce its temperature and moisture content to levels suitable for downstream processing and utilization. Heat exchangers, quenching towers, and condensers may be used to achieve gas cooling and moisture removal.
- Syngas Compression and Storage: The conditioned syngas may undergo compression to increase its pressure for transportation or storage purposes. Depending on the application, compressed syngas may be stored in gas cylinders, tanks, or pipelines for later use or distribution.
- Syngas Utilization Systems: Syngas produced in the gasification plant can be utilized in various energy and chemical processes, including power generation, heat production, transportation fuels, chemical synthesis, and hydrogen production. Utilization systems may include engines, turbines, boilers, fuel cells, catalytic reactors, and chemical conversion units tailored to specific applications.
- Heat Recovery and Integration: Waste heat generated during the gasification process and syngas utilization can be recovered and utilized for process heating, steam generation, or district heating. Heat recovery systems such as heat exchangers, boilers, and cogeneration units improve overall energy efficiency and reduce operational costs.
- Emissions Control and Monitoring: Emissions control systems are installed to monitor and mitigate air pollutants, greenhouse gases, and other emissions generated during biomass gasification and syngas utilization. These systems may include exhaust gas treatment units, scrubbers, catalytic converters, and continuous emissions monitoring systems (CEMS).
- Instrumentation and Control Systems: Biomass gasification plants are equipped with sophisticated instrumentation and control systems to monitor process parameters, control equipment operation, and ensure safe and efficient plant performance. Automation, data acquisition, and remote monitoring technologies optimize plant operation and maintenance.
Biomass gasification plants play a vital role in the transition to renewable energy sources by enabling the conversion of biomass residues and waste materials into clean and sustainable syngas for heat, power, and chemical production. Continuous advancements in gasification technology, process integration, and resource optimization contribute to the development of efficient and environmentally responsible biomass-to-energy solutions.
Gasification Biomass Feedstock:
The choice of biomass feedstock is a critical aspect of gasification processes, as it directly impacts the efficiency, performance, and environmental footprint of the gasification system. Gasification biomass feedstocks are diverse and can include various types of organic materials derived from forestry, agriculture, municipal solid waste, and industrial by-products. Each feedstock has unique characteristics that influence gasification reactor design, process parameters, and syngas composition. Here are some commonly used gasification biomass feedstocks:
- Woody Biomass: Woody biomass comprises materials derived from trees and woody plants, including logs, branches, bark, sawdust, and wood chips. It is one of the most widely used feedstocks for gasification due to its abundance, energy density, and relatively low moisture content. Woody biomass can be sourced from forestry residues, logging operations, urban tree trimmings, and dedicated energy plantations.
- Agricultural Residues: Agricultural residues are by-products of crop production and processing operations, such as straw, rice husks, corn stover, sugarcane bagasse, and wheat chaff. These residues are abundant, renewable, and often available in proximity to gasification facilities, making them attractive feedstocks for bioenergy production. Agricultural residues may require preprocessing to remove impurities and optimize their suitability for gasification.
- Energy Crops: Energy crops are dedicated biomass crops grown specifically for bioenergy production, such as switchgrass, miscanthus, willow, and poplar. These fast-growing, high-yielding crops offer biomass with favorable characteristics for gasification, including high energy content, low moisture content, and minimal mineral content. Energy crops can be cultivated on marginal lands unsuitable for food crops and provide additional ecosystem benefits such as soil erosion control and carbon sequestration.
- Organic Wastes: Organic wastes generated from municipal, industrial, and agricultural activities can serve as valuable feedstocks for gasification, diverting waste from landfills and reducing greenhouse gas emissions. Examples of organic wastes suitable for gasification include sewage sludge, animal manure, food waste, and green waste. Gasification of organic wastes offers a sustainable waste management solution while producing renewable energy and valuable by-products such as biochar and biofuels.
- Algae and Aquatic Biomass: Algae and aquatic biomass, such as microalgae, macroalgae, and aquatic plants, have gained attention as potential feedstocks for bioenergy production due to their rapid growth rates, high lipid content, and ability to grow in diverse aquatic environments. Gasification of algae and aquatic biomass can convert organic matter into syngas while capturing carbon dioxide from the atmosphere, offering a carbon-negative bioenergy pathway.
- Industrial By-products: Various industrial processes generate organic by-products that can be utilized as feedstocks for gasification, including wood residues from lumber mills, paper pulp sludge from paper mills, brewery waste, and distillery residues. Gasification of industrial by-products offers a sustainable approach to waste valorization and energy recovery, reducing disposal costs and environmental impacts associated with waste disposal.
The selection of gasification biomass feedstocks depends on factors such as feedstock availability, cost, quality, logistics, and regional biomass resource potential. Comprehensive feedstock assessments, feedstock supply chain optimization, and feedstock blending strategies are essential for maximizing the economic and environmental sustainability of biomass gasification projects. Additionally, ongoing research and development efforts focus on improving feedstock compatibility, preprocessing technologies, and gasification performance to enhance the viability and scalability of biomass-to-energy conversion technologies.
Biomass Gasification Reactor:
The biomass gasification reactor is a crucial component of gasification systems, where biomass feedstocks undergo thermochemical conversion to produce syngas (synthetic gas). The design and operation of the gasification reactor significantly influence gasification efficiency, syngas composition, and system performance. Several types of gasification reactors are employed in biomass gasification processes, each with unique characteristics and operating principles. Here are some key types of biomass gasification reactors:
- Fixed-Bed Gasifiers: Fixed-bed gasifiers are among the oldest and simplest gasification reactor designs, consisting of a stationary bed of biomass feedstock arranged on a grate or hearth. The biomass undergoes pyrolysis, oxidation, and gasification reactions as it moves downward through the bed, with air or oxygen introduced from below to provide the necessary heat and oxygen for gasification. Fixed-bed gasifiers offer good tar cracking and thermal efficiency but may have limited scalability and operational flexibility.
- Fluidized-Bed Gasifiers: Fluidized-bed gasifiers suspend biomass particles in a bed of inert material (e.g., sand, char) using a high-velocity stream of gas (usually steam or air) flowing from the bottom of the reactor. The fluidized bed provides excellent mixing and heat transfer, promoting rapid gasification reactions and reducing tar formation. Fluidized-bed gasifiers can handle a wide range of feedstocks, including high-moisture materials, and offer good scalability and control over gasification conditions.
- Entrained-Flow Gasifiers: Entrained-flow gasifiers entrain finely ground biomass particles in a high-velocity stream of gas (usually oxygen or air) flowing through the reactor. Biomass particles are rapidly heated and converted to syngas as they travel through the reactor, with residence times typically ranging from milliseconds to seconds. Entrained-flow gasifiers operate at high temperatures and pressures, enabling efficient tar cracking and high syngas yields. They are well-suited for processing high-ash and high-moisture feedstocks but require robust refractory materials and gas cleaning systems.
- Dual Fluidized-Bed Gasifiers: Dual fluidized-bed gasifiers consist of two interconnected fluidized beds: a biomass gasification bed and a bed of inert material (e.g., sand, limestone) acting as a heat carrier. Biomass feedstock is introduced into the gasification bed, where it undergoes partial oxidation and gasification reactions, while heat is supplied by circulating hot solids from the inert bed. Dual fluidized-bed gasifiers offer excellent heat transfer and tar reduction, enabling efficient biomass conversion and syngas production.
- Indirect Gasifiers: Indirect gasifiers employ external heat sources, such as combustion of a portion of the biomass or supplemental fuels, to provide the energy required for gasification. Biomass feedstock is introduced into a reactor chamber where it is heated indirectly by hot gases or surfaces, promoting thermal decomposition and gasification reactions. Indirect gasifiers offer flexibility in fuel selection and can accommodate a wide range of feedstocks and operating conditions.
The selection of a gasification reactor depends on factors such as feedstock properties, desired syngas composition, process requirements, and project economics. Each reactor type has advantages and limitations in terms of efficiency, scalability, feedstock flexibility, and operational complexity. Ongoing research and development efforts focus on improving reactor performance, enhancing gasification kinetics, and developing advanced reactor designs to optimize biomass-to-energy conversion processes.
Gasification Process Control:
Gasification process control encompasses the monitoring, regulation, and optimization of various parameters and operating conditions to ensure efficient and reliable operation of biomass gasification systems. Effective process control is essential for achieving desired syngas composition, maximizing energy conversion efficiency, minimizing emissions, and maintaining system stability. Here are some key aspects of gasification process control:
- Feedstock Preparation and Handling: Proper preparation and handling of biomass feedstocks are critical for maintaining consistent gasification performance. Process control measures include ensuring proper sizing, drying, and conditioning of feedstocks to optimize their reactivity, moisture content, and physical properties. Monitoring feedstock characteristics such as particle size distribution, moisture content, and ash content enables adjustments to feedstock preparation processes to ensure optimal gasification performance.
- Feedstock Metering and Feeding: Accurate metering and feeding of biomass feedstocks into the gasification reactor are essential for maintaining stable gasification conditions and preventing disruptions in syngas production. Process control systems regulate feedstock flow rates, feedstock-to-air ratios, and residence times in the reactor to optimize gasification kinetics and syngas quality. Feedback control loops based on real-time measurements of feedstock flow rates, pressure, temperature, and composition enable precise adjustment of feedstock feeding systems.
- Gasification Reactor Operation: Gasification reactors require careful control of operating parameters such as temperature, pressure, residence time, and gas composition to achieve efficient biomass conversion and syngas production. Process control strategies include maintaining optimal reactor temperatures to promote desired gasification reactions while minimizing tar formation and controlling gasification air/oxygen ratios to ensure complete oxidation of biomass constituents. Advanced control algorithms and automation technologies enable dynamic adjustment of reactor operating conditions based on real-time process measurements and predictive models.
- Syngas Cooling and Cleaning: After exiting the gasification reactor, raw syngas undergoes cooling, cleaning, and conditioning steps to remove impurities, condense tar, and adjust its composition for downstream utilization or storage. Process control systems regulate syngas cooling rates, quenching temperatures, and scrubbing media to achieve efficient tar removal and syngas purification. Monitoring key syngas quality parameters such as tar content, hydrogen/carbon monoxide ratio, and sulfur concentration enables fine-tuning of syngas cleaning processes to meet specific product specifications or environmental regulations.
- Heat and Power Integration: Gasification systems often incorporate combined heat and power (CHP) generation to maximize energy utilization and system efficiency. Process control strategies optimize the integration of gasification reactors with downstream syngas processing units, heat recovery systems, and power generation equipment to maximize overall energy recovery and minimize energy losses. Coordinated control of gasification, syngas cleanup, and power generation units enables dynamic response to fluctuations in feedstock quality, demand, and operating conditions.
- Safety and Emergency Shutdown Systems: Gasification plants implement safety systems and emergency shutdown procedures to protect personnel, equipment, and the environment in case of abnormal operating conditions or process upsets. Process control measures include implementing alarms, interlocks, and automatic shutdown sequences to mitigate risks associated with equipment malfunctions, gas leaks, fires, or overpressure events. Comprehensive risk assessments, safety training programs, and emergency response protocols are essential components of effective gasification process control systems.
Overall, effective process control is essential for optimizing the performance, reliability, and safety of biomass gasification systems, enabling sustainable and cost-effective production of syngas for various applications, including power generation, heat production, biofuels synthesis, and chemical manufacturing. Continuous advancements in control technologies, sensor instrumentation, and data analytics are driving improvements in gasification process control capabilities, enabling enhanced efficiency, flexibility, and resilience of biomass-to-energy conversion processes.
Gasification Tar Removal:
Gasification tar removal is a crucial step in biomass gasification processes aimed at producing clean and high-quality syngas suitable for various energy and chemical applications. Tar, also known as volatile organic compounds (VOCs), is a complex mixture of hydrocarbons and other organic compounds formed during the gasification of biomass feedstocks. Tar can have detrimental effects on downstream equipment, catalysts, and end products, making its removal essential for efficient and reliable gasification operation. Here are some key methods for gasification tar removal:
- Quenching and Condensation: One of the primary methods for tar removal involves rapid cooling and condensation of raw syngas exiting the gasification reactor. Quenching the hot syngas with water or other cooling media causes tar compounds to condense into liquid or solid particles, which can be separated from the gas stream using cyclones, scrubbers, or filters. Quenching and condensation systems are typically integrated into gasification plants to capture tar and other condensable compounds before they can cause fouling or corrosion in downstream equipment.
- Tar Cracking: Tar cracking involves subjecting the raw syngas to high temperatures (>800°C) in the presence of catalysts or reactive surfaces to thermally decompose tar compounds into smaller, more stable molecules such as methane, hydrogen, and carbon monoxide. Tar cracking reactions occur via thermal decomposition, steam reforming, and catalytic reforming mechanisms, with catalysts such as dolomite, nickel, or alkali metals facilitating tar conversion at lower temperatures and residence times. Tar cracking is typically carried out in downstream syngas cleaning units, such as reformers, hot gas filters, or fixed-bed reactors, to enhance syngas quality and reduce tar levels.
- Sorption and Adsorption: Sorption-based methods involve the use of solid sorbents or adsorbents to selectively capture tar compounds from the syngas stream. Activated carbon, molecular sieves, zeolites, and metal oxides are commonly used sorbents with high surface areas and affinity for tar molecules. Syngas is passed through a bed of sorbent material, where tar compounds are adsorbed onto the surface or trapped within the porous structure of the sorbent particles. Regeneration of spent sorbents is achieved by desorption using heat, steam, or vacuum, allowing for cyclic operation of the sorption process. Sorption-based tar removal systems are effective for removing trace amounts of tar and other contaminants from syngas streams with high purity requirements.
- Catalytic Tar Reforming: Catalytic tar reforming involves passing the raw syngas over catalyst surfaces capable of promoting tar cracking and reforming reactions at relatively mild conditions. Catalysts such as nickel, cobalt, iron, and supported metal catalysts are used to catalyze tar decomposition, hydrogenation, and reforming reactions, converting tar compounds into lighter hydrocarbons and gases. Catalytic tar reforming processes can be integrated into gasification reactors or downstream syngas cleaning units to enhance tar conversion efficiency and syngas quality. Catalyst deactivation and poisoning by tar constituents are key challenges in catalytic tar reforming, requiring periodic regeneration or replacement of catalyst materials.
- Hydrodynamic Tar Removal: Hydrodynamic tar removal methods utilize the physical properties of tar droplets, including their size, density, and surface tension, to separate them from the syngas stream using centrifugal, gravitational, or inertial forces. Cyclones, scrubbers, electrostatic precipitators, and impingement separators are commonly used hydrodynamic tar removal devices, which rely on differences in particle inertia, momentum, or electrical charge to capture tar particles entrained in the gas stream. Hydrodynamic tar removal systems are effective for coarse tar removal and particulate matter separation but may require complementary methods for fine tar and aerosol removal.
Overall, gasification tar removal is a complex and multifaceted process that requires careful consideration of operating conditions, feedstock properties, syngas composition, and product specifications. Integrated tar removal systems combining multiple removal mechanisms are often employed to achieve stringent tar content targets and ensure reliable operation of biomass gasification plants for sustainable energy production. Ongoing research and development efforts focus on improving tar removal efficiency, reducing operating costs, and enhancing system reliability through advances in materials, catalysts, process design, and control strategies.
Syngas Cleaning and Conditioning:
Syngas cleaning and conditioning are critical steps in biomass gasification processes to ensure the production of high-quality syngas suitable for downstream utilization in power generation, chemical synthesis, or other industrial applications. Raw syngas produced from biomass gasification typically contains various impurities, including tars, particulate matter, sulfur compounds, moisture, and trace contaminants, which must be removed or reduced to meet product specifications and environmental regulations. Syngas cleaning and conditioning involve a combination of physical, chemical, and thermal treatment methods to achieve the desired syngas composition and purity. Here are some key aspects of syngas cleaning and conditioning:
- Tar Removal: Tar, a complex mixture of hydrocarbons and other organic compounds, is a major impurity in raw syngas produced from biomass gasification. Tar removal is essential to prevent fouling and corrosion of downstream equipment, catalyst deactivation, and adverse effects on syngas quality. Tar removal methods include quenching and condensation, sorption and adsorption, catalytic cracking, and hydrodynamic separation. These methods aim to capture and convert tar compounds into lighter gases or remove them from the syngas stream through physical or chemical processes.
- Particulate Matter Removal: Particulate matter, including ash, char, and soot particles, can entrain in the syngas stream during biomass gasification and cause abrasion, erosion, or fouling in downstream equipment. Particulate matter removal is achieved through mechanical filtration, cyclone separation, electrostatic precipitation, or gravity settling methods. These methods rely on differences in particle size, density, and electrical properties to separate particulate matter from the syngas stream and prevent its accumulation in syngas cleanup and utilization systems.
- Sulfur Removal: Sulfur compounds, such as hydrogen sulfide (H2S) and carbonyl sulfide (COS), are common contaminants in syngas produced from biomass gasification, which can cause corrosion, catalyst poisoning, and environmental emissions. Sulfur removal techniques include chemical scrubbing, adsorption onto metal oxides or activated carbon, and biological desulfurization using specialized microorganisms. These methods aim to selectively remove sulfur compounds from the syngas stream while minimizing energy consumption and waste generation.
- Moisture Removal: Moisture content in syngas affects its heating value, corrosion potential, and downstream processing requirements. Moisture removal is typically achieved through condensation or dehydration processes, such as cooling and water quenching, membrane separation, or desiccant drying. These methods aim to reduce syngas moisture content to levels compatible with downstream utilization or storage requirements while minimizing energy consumption and equipment complexity.
- Contaminant Monitoring and Control: Syngas cleaning and conditioning systems incorporate monitoring and control mechanisms to ensure effective removal of impurities and compliance with product specifications. Online analyzers, sensors, and sampling systems are used to monitor key syngas parameters, including tar content, particulate emissions, sulfur concentration, moisture content, and trace contaminants. Feedback control loops and automated systems enable real-time adjustment of process parameters, such as temperature, pressure, flow rates, and reagent dosing, to optimize syngas cleaning performance and minimize operating costs.
- Integration with Downstream Processes: Syngas cleaning and conditioning systems are integrated with downstream utilization processes, such as gas engines, turbines, fuel cells, or chemical reactors, to ensure compatibility and efficiency of syngas utilization. Syngas quality requirements and process constraints dictate the design and operation of syngas cleanup units, which may vary depending on the specific application and end-user requirements. Comprehensive system integration and optimization are essential to maximize energy recovery, minimize emissions, and ensure reliable operation of biomass gasification plants.
Overall, syngas cleaning and conditioning play a critical role in biomass gasification processes, enabling the production of clean and versatile syngas for sustainable energy production and industrial applications. Continuous research and development efforts focus on improving the efficiency, reliability, and environmental performance of syngas cleanup technologies through advances in materials, catalysts, process design, and control strategies.
Gasification Catalysts:
Gasification catalysts play a crucial role in biomass gasification processes by enhancing reaction rates, promoting syngas quality, and improving process efficiency. These catalysts facilitate the conversion of biomass feedstocks into syngas, a mixture of hydrogen (H2), carbon monoxide (CO), and other gases, which can be used for power generation, fuel synthesis, or chemical production. Catalysts accelerate gasification reactions by lowering activation energies and increasing reaction selectivity, enabling favorable thermodynamic pathways and reducing process operating conditions. Here are some key aspects of gasification catalysts:
- Types of Catalysts: Gasification catalysts encompass a wide range of materials, including transition metals, metal oxides, mixed metal oxides, supported catalysts, and biological catalysts. Transition metals such as nickel (Ni), cobalt (Co), iron (Fe), and ruthenium (Ru) are commonly used for catalyzing gasification reactions due to their high activity, stability, and availability. Metal oxides such as alumina (Al2O3), silica (SiO2), and zirconia (ZrO2) are also used as catalyst supports or promoters to enhance catalytic performance and durability. In addition, biological catalysts, such as enzymes or microorganisms, have been explored for biomass conversion in bio-gasification processes.
- Catalytic Reactions: Gasification catalysts facilitate several key reactions involved in biomass conversion, including pyrolysis, steam reforming, water-gas shift (WGS), methanation, and tar cracking. Pyrolysis reactions involve the thermal decomposition of biomass into volatile gases, char, and tar compounds, which are subsequently converted into syngas components (H2 and CO) through steam reforming and WGS reactions. Methanation reactions convert residual CO and CO2 into methane (CH4), a valuable fuel or chemical precursor, while tar cracking reactions decompose complex hydrocarbons into simpler, more reactive species.
- Catalyst Performance: The performance of gasification catalysts is influenced by various factors, including chemical composition, surface area, porosity, acidity, and thermal stability. Catalysts with high surface area and porosity provide more active sites for gasification reactions and promote better gas-solid interactions. The acidity or basicity of catalyst surfaces affects reaction kinetics and selectivity, with acidic catalysts favoring dehydration and cracking reactions, while basic catalysts promote reforming and methanation reactions. Thermal stability is crucial for maintaining catalytic activity under high-temperature gasification conditions and preventing catalyst deactivation or sintering.
- Catalyst Preparation: Gasification catalysts are typically prepared via impregnation, precipitation, or deposition methods, where active metal species are dispersed onto porous support materials to increase surface area and dispersion. Catalyst synthesis involves several steps, including catalyst precursor preparation, support material selection, metal loading, drying, calcination, and activation. Catalyst properties can be tailored through variation of synthesis parameters such as pH, temperature, mixing ratio, and impregnation method to optimize catalytic performance and stability.
- Catalyst Deactivation: Catalyst deactivation is a common challenge in gasification processes due to fouling, poisoning, or sintering of active sites by tar, ash, sulfur compounds, or trace contaminants present in biomass feedstocks. Tar compounds can adsorb onto catalyst surfaces and block active sites, reducing catalytic activity and selectivity. Ash particles can physically obstruct pore channels or promote catalyst abrasion and attrition, leading to catalyst deactivation over time. Sulfur compounds can poison catalytic sites or promote metal sulfide formation, inhibiting gasification reactions and reducing syngas quality. Strategies to mitigate catalyst deactivation include periodic regeneration, catalyst rejuvenation, catalyst bed management, and feedstock pretreatment to minimize impurity levels.
Gasification catalysts play a vital role in enabling efficient and sustainable biomass conversion technologies for renewable energy production and carbon mitigation. Ongoing research and development efforts focus on advancing catalyst synthesis techniques, improving catalytic performance and durability, enhancing catalyst-substrate interactions, and tailoring catalyst formulations for specific gasification applications and feedstock compositions. Collaboration between academia, industry, and government agencies is essential to accelerate the commercialization and deployment of advanced gasification catalysts for global energy transition and environmental stewardship.
Syngas Utilization:
Syngas utilization is a key aspect of biomass gasification processes, where the produced syngas, a mixture of hydrogen (H2) and carbon monoxide (CO), is converted into valuable products such as electricity, fuels, chemicals, and heat. Syngas, rich in energy content and versatile in applications, serves as a valuable intermediate for various industrial processes and energy systems. Syngas utilization pathways are diverse and tailored to specific end-use requirements, economic considerations, and environmental goals. Here are some key aspects of syngas utilization:
- Electricity Generation: One of the primary applications of syngas is electricity generation through combustion in gas turbines, reciprocating engines, or fuel cells. Syngas can replace natural gas or diesel fuel in existing power generation facilities with minor modifications to combustion equipment. Gas turbines and reciprocating engines are commonly used for syngas-based power generation due to their high efficiency, fast startup, and grid compatibility. Fuel cells offer higher efficiency and lower emissions but require additional infrastructure and capital investment.
- Fuels Production: Syngas can be further processed to produce a wide range of fuels, including hydrogen (H2), methane (CH4), synthetic natural gas (SNG), diesel, gasoline, and aviation fuels. Syngas can undergo catalytic processes such as Fischer-Tropsch synthesis, methanol synthesis, or hydrocarbon reforming to produce liquid fuels or gaseous fuels with properties similar to conventional fossil fuels. These synthetic fuels can be blended with existing fuels or used directly in transportation, heating, and industrial applications, offering carbon-neutral alternatives to fossil-derived fuels.
- Chemical Synthesis: Syngas serves as a feedstock for chemical synthesis processes, enabling the production of various commodity chemicals, intermediates, and specialty products. Chemical processes such as ammonia synthesis, methanol synthesis, oxo synthesis, and hydrogenation reactions utilize syngas as a precursor to produce fertilizers, plastics, solvents, polymers, pharmaceuticals, and fine chemicals. These chemical products have diverse industrial applications and contribute to the production of essential goods for society.
- Hydrogen Production: Syngas can be directly converted into hydrogen (H2) through water-gas shift (WGS) reactions or membrane separation processes. Hydrogen is a clean and versatile energy carrier used in industrial processes, fuel cells, transportation, and energy storage applications. Syngas-derived hydrogen can be used to decarbonize various sectors, including refineries, petrochemicals, steelmaking, and ammonia production, where hydrogen serves as a reducing agent or chemical feedstock.
- Heat and Steam Generation: Syngas can be combusted directly to produce heat or steam for industrial processes, district heating, or cogeneration applications. Syngas-based heat and steam generation systems utilize waste heat recovery technologies to improve overall energy efficiency and reduce environmental emissions. These systems provide thermal energy for industrial processes, space heating, hot water production, and steam-driven power generation in combined heat and power (CHP) plants.
Syngas utilization technologies continue to evolve to meet the growing demand for clean, sustainable, and cost-effective energy solutions. Advancements in gasification processes, catalyst development, process integration, and system optimization contribute to the commercialization and deployment of syngas-based energy systems worldwide. Syngas utilization offers significant opportunities for energy diversification, carbon mitigation, and economic development, supporting the transition towards a more sustainable and resilient energy future.
Gasification Plant Design:
Gasification plant design involves the comprehensive engineering and integration of equipment, systems, and processes to convert biomass feedstocks into syngas efficiently and reliably. Gasification plants are complex systems comprising various unit operations, reactors, heat exchangers, gas cleaning devices, and control systems to facilitate biomass conversion and syngas production. Plant design considerations encompass technical, economic, environmental, and safety aspects to ensure optimal performance and compliance with regulatory requirements. Here are some key aspects of gasification plant design:
- Feedstock Handling and Preparation: Gasification plants are designed to accommodate a wide range of biomass feedstocks, including wood chips, agricultural residues, energy crops, municipal solid waste (MSW), and organic wastes. Feedstock handling systems include storage silos, feeders, conveyors, and size reduction equipment to ensure consistent feedstock supply, particle size distribution, and moisture content. Feedstock properties such as bulk density, moisture content, ash content, and calorific value influence plant design and operation.
- Gasification Reactors: Gasification reactors are the heart of gasification plants, where biomass feedstocks are thermally converted into syngas under controlled conditions of temperature, pressure, and residence time. Various types of gasifiers are used in gasification plants, including fixed-bed, fluidized-bed, entrained-flow, and downdraft gasifiers, each with distinct operating principles, advantages, and limitations. Reactor design considerations include reactor geometry, material selection, refractory lining, gas-solid contacting mechanisms, and tar cracking mechanisms.
- Syngas Cleanup and Conditioning: Syngas produced from gasification reactors contains impurities such as tar, particulates, sulfur compounds, ammonia, and trace contaminants that must be removed to meet product specifications and environmental regulations. Gas cleanup systems include cyclones, filters, scrubbers, condensers, and adsorption units to remove solid particles, tar compounds, acidic gases, and other contaminants from syngas streams. Syngas conditioning processes such as cooling, compression, and moisture removal are also integrated into gasification plant design to optimize syngas properties for downstream utilization or storage.
- Heat Recovery and Integration: Gasification plants employ heat recovery and integration strategies to maximize energy efficiency and minimize environmental impact. Waste heat from gasification processes is recovered through heat exchangers, steam boilers, or thermal oil systems to generate steam, hot water, or electricity for internal plant use or external applications. Heat integration involves optimizing heat transfer networks, process streams, and utility systems to minimize energy consumption, reduce greenhouse gas emissions, and enhance overall plant performance.
- Instrumentation and Control Systems: Gasification plants are equipped with advanced instrumentation and control systems to monitor process parameters, control operating conditions, and ensure safe and reliable plant operation. Supervisory control and data acquisition (SCADA) systems, distributed control systems (DCS), and programmable logic controllers (PLC) are used to automate process control loops, regulate feed rates, adjust operating conditions, and respond to process disturbances. Safety systems such as emergency shutdown (ESD) systems, fire detection systems, and gas detection systems are also integrated into gasification plant design to protect personnel, equipment, and the environment.
Gasification plant design is a multidisciplinary endeavor that requires expertise in process engineering, mechanical engineering, chemical engineering, electrical engineering, instrumentation engineering, and safety engineering. Collaboration between engineering disciplines, project stakeholders, equipment suppliers, and regulatory agencies is essential to ensure successful project execution and achieve project objectives in terms of cost, schedule, performance, and sustainability. Gasification plants play a critical role in the transition to a low-carbon economy by enabling the conversion of renewable biomass resources into clean and versatile syngas for power generation, fuels production, and chemical synthesis.
Gas Engine
- Combustion: Gas engines utilize combustion of fuel-air mixtures to produce mechanical energy through the expansion of high-pressure gases within the engine cylinders.
- Internal Combustion Engine: Gas engines are a type of internal combustion engine (ICE) that converts the chemical energy of fuel into mechanical energy through combustion within the engine cylinders.
- Natural Gas: Gas engines are commonly fueled by natural gas, a clean-burning fossil fuel composed primarily of methane (CH4) extracted from underground reservoirs.
- Biogas: Gas engines can also be fueled by biogas, a renewable energy source produced from the anaerobic digestion of organic waste materials such as agricultural residues, municipal solid waste (MSW), and wastewater sludge.
- Syngas: Gas engines can operate on syngas, a synthetic gas mixture composed of hydrogen (H2) and carbon monoxide (CO) produced from biomass gasification processes.
- Fuel Flexibility: Gas engines offer fuel flexibility, allowing them to operate on a wide range of gaseous fuels, including natural gas, biogas, landfill gas, sewage gas, and syngas.
- Spark Ignition: Gas engines typically employ spark ignition systems to initiate combustion by igniting the fuel-air mixture with an electric spark generated by spark plugs.
- Compression Ignition: Some gas engines utilize compression ignition systems, also known as lean-burn or dual-fuel engines, where combustion is initiated by compressing the fuel-air mixture to high pressures and temperatures.
- Cylinder Arrangement: Gas engines may have different cylinder arrangements, including inline, V-type, horizontally opposed (boxer), and radial configurations, depending on the application and power output requirements.
- Turbocharging: Turbocharged gas engines use exhaust gas-driven turbochargers to compress incoming air, increasing the air-fuel mixture’s density and improving engine performance and efficiency.
- Intercooling: Intercooled gas engines incorporate intercoolers to cool the compressed air before entering the combustion chambers, reducing the risk of knock and improving engine efficiency.
- Lean-Burn Technology: Lean-burn gas engines operate with excess air in the combustion chamber, resulting in lower combustion temperatures, reduced emissions, and improved fuel efficiency compared to stoichiometric engines.
- Cogeneration: Gas engines are commonly used in combined heat and power (CHP) systems, where they simultaneously generate electricity and heat for residential, commercial, industrial, and institutional applications.
- Waste Heat Recovery: Gas engines can be equipped with waste heat recovery systems to capture and utilize exhaust heat for space heating, water heating, absorption chilling, or additional power generation.
- Remote Power Generation: Gas engines are employed in remote power generation applications, such as off-grid communities, remote industrial sites, oil and gas fields, and mobile power units, providing reliable electricity supply in areas with limited access to the grid.
- Microgrid Integration: Gas engines play a key role in microgrid systems, providing distributed generation capacity, grid stability support, and resilience against power outages in decentralized energy networks.
- Combined Cycle: Gas engines can be integrated into combined cycle power plants, where waste heat from the engine exhaust is used to generate steam for driving steam turbines, further increasing overall plant efficiency.
- Emissions Control: Gas engines are equipped with emissions control technologies, such as catalytic converters, selective catalytic reduction (SCR) systems, and lean NOx traps, to reduce emissions of nitrogen oxides (NOx), carbon monoxide (CO), and hydrocarbons (HC).
- Maintenance: Gas engines require regular maintenance, including oil and filter changes, spark plug replacement, valve adjustments, and inspection of cooling, fuel, and ignition systems, to ensure reliable operation and optimal performance.
- Remote Monitoring: Gas engines can be equipped with remote monitoring and diagnostic systems that enable real-time performance monitoring, fault detection, and predictive maintenance scheduling, maximizing uptime and reducing operational costs.
Combustion:
Combustion is a fundamental process in gas engines where a fuel-air mixture undergoes rapid oxidation, releasing energy in the form of heat that is converted into mechanical work. In gas engines, combustion occurs within the engine cylinders, where a precisely metered mixture of fuel and air is ignited to produce power.
The combustion process begins with the intake stroke, where the piston moves downward, drawing in a mixture of fuel and air into the cylinder through the intake valve. This mixture is then compressed during the compression stroke as the piston moves upward, reducing its volume and increasing its pressure and temperature. At the top dead center of the compression stroke, the spark plug ignites the compressed mixture, initiating combustion.
Once ignited, the fuel-air mixture undergoes rapid combustion, with the chemical energy of the fuel being converted into thermal energy. This sudden increase in pressure and temperature forces the piston downward during the power stroke, generating mechanical work that drives the crankshaft and ultimately powers the vehicle or machinery.
The combustion process is highly dependent on factors such as the fuel-air ratio, ignition timing, compression ratio, and turbulence within the combustion chamber. Proper combustion is essential for achieving maximum efficiency, power output, and emissions control in gas engines.
In summary, combustion is the process by which fuel is burned in the presence of oxygen to release energy, driving the operation of gas engines and providing power for various applications. Understanding and optimizing combustion processes are crucial for improving engine performance, efficiency, and environmental sustainability.
Internal Combustion Engine:
An internal combustion engine (ICE) is a type of heat engine that generates mechanical power by burning fuel within its cylinders. Gas engines are a specific type of internal combustion engine that uses gaseous fuels such as natural gas, biogas, or syngas as the primary fuel source.
The operation of an internal combustion engine involves four main strokes: intake, compression, power, and exhaust. During the intake stroke, the engine’s intake valve opens, allowing a mixture of fuel and air to enter the combustion chamber. In the compression stroke, the intake valve closes, and the piston compresses the fuel-air mixture, increasing its pressure and temperature. The compression stroke ends with the ignition of the compressed mixture by a spark plug or through compression ignition.
The ignited mixture undergoes rapid combustion, producing high-pressure gases that force the piston downward during the power stroke, generating mechanical work. Finally, during the exhaust stroke, the exhaust valve opens, and the piston pushes the remaining exhaust gases out of the cylinder.
Gas engines are known for their versatility, fuel efficiency, and lower emissions compared to other types of internal combustion engines. They are widely used in various applications, including vehicles (such as cars, buses, and trucks), stationary power generation (such as generators and cogeneration systems), and industrial equipment (such as pumps, compressors, and construction machinery).
Gas engines offer several advantages, including lower fuel costs, reduced greenhouse gas emissions, and quieter operation compared to diesel engines. However, they also require specific fuel storage and delivery systems, as well as regular maintenance to ensure optimal performance and reliability.
Overall, gas engines play a significant role in modern transportation, power generation, and industrial processes, offering efficient and environmentally friendly solutions for meeting energy needs. Continued research and development in gas engine technology are essential for further improving efficiency, reducing emissions, and expanding their applications in the transition to a more sustainable energy future.
Natural Gas:
Natural gas is a versatile fossil fuel consisting primarily of methane (CH4) with varying amounts of other hydrocarbons, such as ethane, propane, and butane, as well as small quantities of impurities like nitrogen, carbon dioxide, and hydrogen sulfide. It is found in underground reservoirs formed by the decomposition of organic matter over millions of years.
One of the key advantages of natural gas is its relatively clean-burning nature compared to other fossil fuels. When combusted, natural gas emits fewer pollutants such as sulfur dioxide (SO2), nitrogen oxides (NOx), particulate matter, and carbon monoxide (CO) compared to coal or oil. This makes it a preferred fuel choice for applications where emissions reduction is a priority, such as power generation, heating, and transportation.
In addition to its environmental benefits, natural gas is also abundant and widely distributed around the world, making it a reliable and accessible energy source. It is extracted from underground reservoirs using drilling techniques similar to those used in oil exploration and production.
Natural gas is used in various sectors and applications, including:
- Power Generation: Natural gas-fired power plants produce electricity by burning natural gas to heat water and produce steam, which drives turbines connected to generators. These power plants are known for their high efficiency, fast startup times, and lower emissions compared to coal-fired plants.
- Heating: Natural gas is commonly used for residential, commercial, and industrial heating applications, including space heating, water heating, and process heating. It is distributed to end-users through pipelines and is often favored for its convenience, cleanliness, and cost-effectiveness.
- Transportation: Natural gas can be used as a transportation fuel in the form of compressed natural gas (CNG) or liquefied natural gas (LNG). It is used in buses, trucks, and fleet vehicles, offering lower emissions and reduced fuel costs compared to traditional gasoline or diesel fuels.
- Industrial Applications: Natural gas is used as a fuel and feedstock in various industrial processes, including chemical production, metal smelting, glass manufacturing, and food processing. It provides reliable and efficient energy for heating, drying, and powering industrial equipment.
- Cogeneration: Natural gas is often used in combined heat and power (CHP) systems, where it simultaneously generates electricity and useful heat for heating or cooling applications. CHP systems are highly efficient and can help reduce energy costs and greenhouse gas emissions.
Overall, natural gas plays a crucial role in meeting global energy demand, providing a cleaner and more sustainable alternative to traditional fossil fuels. Continued advancements in natural gas technology, exploration, and production will be essential for maximizing its benefits while minimizing environmental impacts.
Syngas:
Syngas, short for synthesis gas, is a fuel gas mixture consisting primarily of hydrogen (H2) and carbon monoxide (CO), along with varying amounts of other gases such as carbon dioxide (CO2), methane (CH4), and nitrogen (N2). It is produced through the gasification of carbon-containing feedstocks such as coal, biomass, or municipal solid waste.
The production of syngas typically involves the partial oxidation or steam reforming of the feedstock in a gasifier, a high-temperature reactor where the feedstock is converted into a gaseous mixture of hydrogen and carbon monoxide. The composition of syngas can be adjusted by varying the operating conditions of the gasifier, such as temperature, pressure, and the ratio of steam to carbon.
Syngas has a wide range of applications across various industries, including:
- Power Generation: Syngas can be burned directly in gas turbines or used as fuel in internal combustion engines to generate electricity. It is often used in combined cycle power plants, where the waste heat from power generation is recovered and used to produce steam for additional electricity generation.
- Chemical Synthesis: Syngas serves as a feedstock for the production of a wide range of chemicals and fuels through processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia synthesis. These processes convert syngas into valuable products such as synthetic fuels, hydrogen, ammonia, methanol, and synthetic natural gas (SNG).
- Hydrogen Production: Syngas can be further processed to produce pure hydrogen gas through processes such as water-gas shift reaction or membrane separation. Hydrogen is an important industrial gas used in various applications, including petroleum refining, chemical production, and fuel cells.
- Bioenergy: Syngas produced from biomass feedstocks such as wood, agricultural residues, or organic waste can be used as a renewable energy source. Biomass gasification with syngas production offers an alternative to traditional combustion-based biomass energy systems and can provide a sustainable solution for power generation and heating.
- Waste Treatment: Syngas can be produced from the gasification of municipal solid waste, sewage sludge, or industrial waste streams. Gasification offers a more environmentally friendly alternative to landfilling or incineration, as it can convert waste materials into useful energy products while minimizing environmental impacts.
Overall, syngas is a versatile energy carrier with the potential to play a significant role in the transition to a more sustainable and low-carbon energy future. Continued research and development in syngas production and utilization technologies will be essential for unlocking its full potential and maximizing its benefits across various sectors.
Gasification:
Gasification is a thermochemical conversion process that converts carbonaceous feedstocks, such as coal, biomass, or municipal solid waste, into a gaseous mixture called syngas (synthesis gas). This process involves the partial oxidation of the feedstock at high temperatures (>700°C) and controlled oxygen levels in the presence of steam or a combination of oxygen and steam.
The gasification process typically occurs in a gasifier, which is a high-temperature reactor where the feedstock is subjected to heat, pressure, and a controlled environment to promote the chemical reactions that produce syngas. The key reactions involved in gasification include:
- Pyrolysis: The feedstock is heated in an oxygen-starved environment, causing it to decompose into volatile compounds, char, and ash. The volatile compounds undergo further decomposition to form gases such as methane, ethylene, and hydrogen.
- Oxidation: Oxygen or steam is introduced into the gasifier to react with the carbonaceous materials in the feedstock, converting them into carbon monoxide and hydrogen through partial oxidation reactions. These reactions are exothermic and provide the heat necessary to sustain the gasification process.
- Water-Gas Shift Reaction: Carbon monoxide produced during the oxidation stage reacts with steam to form additional hydrogen and carbon dioxide through the water-gas shift reaction. This reaction helps increase the hydrogen content of the syngas and improve its energy content.
- Methanation: In some gasification processes, carbon dioxide and residual hydrogen may react to form methane through methanation reactions. Methanation can help improve the energy efficiency of the gasification process by producing a higher-quality fuel gas.
The resulting syngas produced from gasification typically contains hydrogen, carbon monoxide, carbon dioxide, methane, and other trace gases, depending on the feedstock and gasification conditions. This syngas can be used as a versatile fuel for various applications, including power generation, chemical synthesis, hydrogen production, and bioenergy.
Gasification offers several advantages over traditional combustion-based processes, including higher efficiency, lower emissions, and greater fuel flexibility. It enables the conversion of a wide range of feedstocks into valuable energy products while minimizing environmental impacts. Additionally, gasification facilitates the production of syngas from renewable biomass sources, contributing to the development of sustainable energy solutions.
However, gasification also presents technical challenges, such as feedstock preparation, tar and ash management, and syngas cleanup. Continued research and development efforts are focused on addressing these challenges and optimizing gasification technologies for commercial-scale deployment in various industries. Overall, gasification holds great potential as a clean and efficient pathway for converting carbonaceous materials into valuable energy resources.
Biomass Gasification:
Biomass gasification is a thermochemical process that converts biomass feedstocks into a combustible gas mixture known as syngas (synthesis gas), which primarily consists of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and methane (CH4). This process occurs in a gasifier, a high-temperature reactor where biomass is heated in a controlled environment with limited oxygen or air supply.
The biomass feedstock, which can include various organic materials such as wood chips, agricultural residues, or energy crops, undergoes several chemical reactions during gasification:
- Drying: Initially, the moisture content of the biomass is reduced through drying to improve the efficiency of the gasification process. Excess moisture can consume energy and reduce the temperature inside the gasifier.
- Pyrolysis: As the temperature increases in the gasifier, the biomass undergoes pyrolysis, a process where organic materials decompose into volatile gases, solid char, and ash. The volatile gases released during pyrolysis include tar, methane, hydrogen, and other hydrocarbons.
- Gasification: The pyrolysis products then react with a limited supply of oxygen or steam in the gasifier to produce syngas through a series of gasification reactions. These reactions typically involve the partial oxidation of carbonaceous materials in the biomass, resulting in the formation of hydrogen and carbon monoxide.
- Tar and Ash Removal: Tar and ash are byproducts of the gasification process and can potentially cause operational issues such as equipment fouling and corrosion. Therefore, tar removal and ash management systems are often incorporated into biomass gasification systems to ensure stable and efficient operation.
Biomass gasification offers several advantages over traditional combustion-based biomass energy systems, including higher energy efficiency, lower emissions, and greater flexibility in feedstock selection. Syngas produced from biomass gasification can be used for various energy applications, including:
- Power Generation: Syngas can be combusted in gas turbines, internal combustion engines, or boilers to generate electricity. Combined heat and power (CHP) systems can further improve overall energy efficiency by utilizing waste heat for heating or cooling purposes.
- Biofuel Production: Syngas can serve as a precursor for the production of liquid biofuels such as ethanol, methanol, or synthetic diesel through catalytic processes such as Fischer-Tropsch synthesis.
- Hydrogen Production: Syngas can be further processed to produce hydrogen gas, which can be used as a clean fuel for transportation, industrial processes, or fuel cells.
Biomass gasification technology continues to advance, with ongoing research focused on improving process efficiency, reducing capital and operating costs, and expanding the range of biomass feedstocks that can be utilized. As a renewable and carbon-neutral energy source, biomass gasification plays a crucial role in the transition to a more sustainable and low-carbon energy future.
Gasification Plant:
A gasification plant is a facility designed to convert carbonaceous feedstocks, such as coal, biomass, or municipal solid waste, into a gaseous fuel known as syngas (synthesis gas) through the process of gasification. These plants typically consist of several integrated components that work together to convert the feedstock into syngas efficiently and reliably.
Key components of a gasification plant include:
- Feedstock Handling System: This system is responsible for receiving, storing, and preparing the feedstock for gasification. Depending on the type of feedstock used, it may involve processes such as shredding, drying, and size reduction to optimize gasification performance.
- Gasifier: The gasifier is the heart of the gasification plant, where the actual conversion of feedstock into syngas takes place. It is a high-temperature reactor that operates in a controlled environment with limited oxygen or air supply to promote the partial oxidation of the feedstock. Gasifiers can be of various types, including fixed-bed, fluidized-bed, or entrained-flow gasifiers, each offering different advantages in terms of efficiency, flexibility, and scalability.
- Gas Cleanup System: The syngas produced in the gasifier contains impurities such as tar, particulates, sulfur compounds, and other contaminants that need to be removed to meet quality specifications for downstream applications. The gas cleanup system typically includes processes such as scrubbing, filtration, catalytic conversion, and chemical treatment to purify the syngas and remove harmful components.
- Syngas Conditioning and Cooling: After cleanup, the syngas may undergo further conditioning to adjust its temperature, pressure, and composition to meet specific requirements for utilization or storage. Additionally, the syngas is cooled to reduce its temperature before being sent to downstream processes or storage facilities.
- Heat Recovery System: Gasification is an energy-intensive process that generates significant amounts of waste heat, which can be recovered and utilized to improve overall energy efficiency. Heat recovery systems may include heat exchangers, boilers, or steam turbines that capture and utilize waste heat for power generation or process heating.
- Syngas Utilization: The purified and conditioned syngas can be utilized for various energy applications, including power generation, chemical synthesis, hydrogen production, and biofuel production. Depending on the specific requirements and market demand, gasification plants may incorporate different downstream processes and equipment to maximize the value of the syngas produced.
Gasification plants offer several advantages over traditional combustion-based energy systems, including higher efficiency, lower emissions, and greater fuel flexibility. They enable the conversion of a wide range of feedstocks into valuable energy products while minimizing environmental impacts. As the demand for clean and sustainable energy continues to grow, gasification technology plays a crucial role in meeting these challenges and advancing towards a more sustainable energy future.
Syngas Production:
Syngas production is a key process in gasification technology, where carbonaceous feedstocks such as coal, biomass, or municipal solid waste are converted into a gaseous mixture known as syngas (synthesis gas). Syngas is a versatile fuel that contains hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and other trace gases, depending on the feedstock and gasification conditions.
The production of syngas typically involves several stages within a gasification plant:
- Feedstock Preparation: The feedstock undergoes preprocessing to remove contaminants, moisture, and impurities that can affect gasification performance. Depending on the feedstock type, preprocessing may include shredding, drying, grinding, and pelletizing to achieve uniform particle size and composition.
- Gasification Reaction: The prepared feedstock is fed into a gasifier, a high-temperature reactor where gasification reactions occur in a controlled environment. The gasifier operates at elevated temperatures (>700°C) and limited oxygen or air supply to promote the partial oxidation of the feedstock. Key gasification reactions include:
- Pyrolysis: Thermal decomposition of the feedstock into volatile gases, char, and ash.
- Reduction: Conversion of carbonaceous materials into carbon monoxide (CO) and hydrogen (H2) through partial oxidation reactions.
- Water-Gas Shift: Conversion of CO and steam into additional hydrogen (H2) and carbon dioxide (CO2) through the water-gas shift reaction.
- Syngas Cleanup: The syngas produced in the gasifier contains impurities such as tar, particulates, sulfur compounds, and other contaminants that need to be removed to meet quality specifications for downstream applications. Syngas cleanup processes include scrubbing, filtration, catalytic conversion, and chemical treatment to purify the syngas and remove harmful components.
- Syngas Conditioning: The purified syngas may undergo further conditioning to adjust its temperature, pressure, and composition to meet specific requirements for utilization or storage. Syngas conditioning processes may include cooling, compression, and adjustment of gas composition to optimize its energy content.
- Syngas Utilization: The conditioned syngas can be utilized for various energy applications, including power generation, chemical synthesis, hydrogen production, and biofuel production. Depending on the specific requirements and market demand, syngas can be combusted directly in gas turbines, internal combustion engines, or boilers, or further processed to produce value-added products such as liquid fuels, chemicals, or hydrogen gas.
Syngas production plays a crucial role in enabling the conversion of carbonaceous feedstocks into valuable energy products while minimizing environmental impacts. As the demand for clean and sustainable energy continues to grow, syngas production technologies such as gasification are expected to play an increasingly important role in meeting these challenges and advancing towards a more sustainable energy future.
Gasification Reactor:
The gasification reactor is the core component of a gasification system where the conversion of carbonaceous feedstock into syngas (synthesis gas) occurs. It is a high-temperature vessel designed to operate under controlled conditions to promote the partial oxidation and thermal decomposition of the feedstock.
The gasification reactor facilitates several key processes essential for syngas production:
- Feedstock Introduction: The carbonaceous feedstock, which can include coal, biomass, or municipal solid waste, is introduced into the gasification reactor. Depending on the reactor design, the feedstock may be fed in the form of solid particles, slurry, or as a fluidized bed.
- Pyrolysis: As the feedstock is heated in the gasification reactor, it undergoes pyrolysis, a thermochemical process where organic materials decompose into volatile gases, solid char, and ash. Pyrolysis occurs at elevated temperatures (>700°C) in the absence of oxygen or with limited oxygen supply, leading to the release of volatiles such as tar, methane, hydrogen, and other hydrocarbons.
- Partial Oxidation: In the presence of a controlled amount of oxygen or steam, the volatile gases produced during pyrolysis undergo partial oxidation reactions to generate syngas. These reactions typically involve the conversion of carbonaceous materials into carbon monoxide (CO) and hydrogen (H2) through endothermic and exothermic reactions:
- Endothermic Reactions: (e.g., C + H2O → CO + H2)
- Exothermic Reactions: (e.g., C + O2 → CO2)
- Temperature Control: The gasification reactor maintains elevated temperatures necessary for pyrolysis and gasification reactions to occur efficiently. Temperature control is critical to ensuring proper reaction kinetics and syngas composition. Various heating methods, such as direct combustion, indirect heating, or plasma heating, may be employed depending on the reactor design and feedstock characteristics.
- Residence Time: The residence time of the feedstock within the gasification reactor influences the extent of pyrolysis and gasification reactions. Optimizing residence time ensures thorough conversion of the feedstock into syngas while minimizing the formation of undesired byproducts such as tar and char.
Gasification reactors can be of different configurations, including fixed-bed, fluidized-bed, entrained-flow, or plasma reactors, each offering unique advantages in terms of reaction kinetics, heat transfer, scalability, and feedstock flexibility. The selection of the appropriate reactor type depends on factors such as feedstock properties, desired syngas composition, throughput requirements, and process economics.
Overall, the gasification reactor plays a critical role in facilitating the efficient conversion of carbonaceous feedstocks into syngas, a versatile energy carrier used in various industrial processes, power generation, and biofuel production. Continuous advancements in reactor design and operation are essential for improving gasification efficiency, reducing environmental impacts, and enhancing the competitiveness of gasification technology in the transition towards a more sustainable energy future.
Gas Cleanup System:
The gas cleanup system is an integral component of gasification technology, responsible for purifying the syngas (synthesis gas) produced during the gasification process. Syngas typically contains impurities such as tar, particulates, sulfur compounds, ammonia, and other contaminants that need to be removed to meet quality specifications for downstream applications.
The gas cleanup system consists of several unit operations designed to remove specific impurities from the syngas stream:
- Particulate Removal: Particulate matter, such as ash, char, and dust, is removed from the syngas stream using mechanical filtration techniques. Cyclone separators, bag filters, or electrostatic precipitators are commonly employed to capture particulates and prevent their entry into downstream equipment.
- Tar Removal: Tar, a complex mixture of organic compounds formed during pyrolysis and gasification, is a major impurity in syngas that can cause fouling and corrosion in downstream equipment. Tar removal is typically achieved through processes such as condensation, scrubbing, or catalytic conversion. Tar-laden syngas is cooled to condense the tar compounds, which can then be separated from the gas stream using separators or scrubbers.
- Sulfur Removal: Sulfur compounds, including hydrogen sulfide (H2S) and carbonyl sulfide (COS), are undesirable impurities in syngas due to their corrosive nature and potential environmental impact. Sulfur removal is accomplished through processes such as desulfurization using chemical absorbents or adsorbents, hydrodesulfurization using catalytic reactors, or biological methods.
- Ammonia Removal: Ammonia (NH3) is often present in syngas derived from biomass feedstocks and can cause catalyst poisoning in downstream processes. Ammonia removal is typically achieved through processes such as selective catalytic reduction (SCR), where ammonia reacts with a catalyst to form nitrogen and water, or absorption using acidic solutions.
- Particulate Removal: In addition to particulate matter, syngas may contain trace amounts of heavy metals, such as mercury, arsenic, and lead, which are harmful to human health and the environment. Heavy metal removal is accomplished through processes such as adsorption onto activated carbon or chemical precipitation.
- Water Removal: Water vapor present in the syngas stream can condense and cause corrosion in downstream equipment. Water removal is typically achieved through processes such as condensation, membrane separation, or dehydration using desiccants.
The selection of gas cleanup technologies depends on factors such as the composition of the syngas, impurity levels, desired syngas quality, and economic considerations. Gas cleanup systems are essential for ensuring the purity of syngas and optimizing its utilization in various industrial processes, power generation, and biofuel production. Continuous advancements in gas cleanup technology are crucial for improving efficiency, reducing operating costs, and enhancing the environmental performance of gasification systems.
Gasification Plant Integration:
Gasification plant integration refers to the process of incorporating gasification technology into existing or new energy systems to maximize efficiency, flexibility, and overall performance. It involves the seamless integration of gasification units with other process units, equipment, and infrastructure to create integrated energy systems capable of utilizing syngas for various applications.
Key aspects of gasification plant integration include:
- Feedstock Handling and Preparation: Gasification plants must be designed to handle a wide range of feedstocks, including coal, biomass, municipal solid waste, and waste plastics. Integration involves optimizing feedstock handling systems, such as feedstock storage, drying, size reduction, and blending, to ensure consistent feedstock quality and availability for the gasification process.
- Gasification Reactor Integration: Gasification reactors are integrated into the overall plant design to optimize process performance and energy efficiency. This includes selecting the appropriate gasification technology (e.g., fixed-bed, fluidized-bed, entrained-flow) and configuring the reactor design to meet specific process requirements and operating conditions.
- Syngas Cleanup and Conditioning: Integrated gas cleanup systems are implemented to remove impurities from the syngas produced by the gasification process. This may involve integrating gas cleanup technologies such as particulate removal, tar removal, sulfur removal, and ammonia removal into the overall plant design to ensure compliance with syngas quality specifications.
- Syngas Utilization: Integrated gasification plants are designed to efficiently utilize the syngas produced for various applications, including power generation, heat production, hydrogen production, and synthesis of chemicals and fuels. Syngas utilization pathways are optimized based on factors such as syngas composition, energy demand, market opportunities, and environmental considerations.
- Waste Heat Recovery: Gasification plants often generate waste heat during the gasification process, which can be recovered and utilized for cogeneration or district heating applications. Integration of waste heat recovery systems allows for the efficient utilization of thermal energy and improves overall plant efficiency.
- Emissions Control and Environmental Compliance: Integration of emissions control technologies, such as selective catalytic reduction (SCR), electrostatic precipitators (ESP), and flue gas desulfurization (FGD), ensures compliance with environmental regulations and minimizes the environmental impact of gasification plant operations.
- Process Optimization and Control: Integration involves implementing advanced process control systems and optimization strategies to maximize plant efficiency, reliability, and flexibility. This includes real-time monitoring, data analytics, and control algorithms to optimize process parameters, minimize operating costs, and respond to changing operating conditions.
Gasification plant integration requires multidisciplinary expertise in engineering, process design, equipment selection, and project management. Successful integration involves thorough planning, detailed engineering, rigorous testing, and commissioning to ensure the seamless operation of the integrated energy system. Continuous monitoring, maintenance, and optimization are essential to maximize the performance and longevity of gasification plants integrated into complex energy systems.
Biomass Gasification Plant:
A biomass gasification plant is a facility designed to convert biomass feedstocks into a combustible gas mixture known as syngas, which can be used as a renewable energy source for various applications, including heat and power generation, biofuels production, and chemical synthesis. The process of biomass gasification involves thermochemical conversion, where biomass is reacted at elevated temperatures in a controlled environment to produce syngas composed mainly of carbon monoxide (CO), hydrogen (H2), and methane (CH4), along with other trace gases.
Key components and processes of a biomass gasification plant include:
- Feedstock Handling and Preparation: Biomass feedstocks, such as wood chips, agricultural residues, energy crops, and organic waste, are transported to the gasification plant and undergo preprocessing operations, including drying, size reduction (e.g., chipping, shredding), and moisture control to optimize feedstock characteristics for gasification.
- Gasification Reactor: The gasification reactor is the heart of the plant, where biomass feedstock undergoes thermochemical conversion in the presence of a controlled amount of oxygen (or steam) and heat. Various gasification technologies, such as fixed-bed, fluidized-bed, and entrained-flow gasifiers, are employed to achieve different operating conditions and syngas compositions.
- Gas Cleanup System: The syngas produced by the gasification process contains impurities such as tar, particulates, sulfur compounds, ammonia, and moisture, which need to be removed to meet quality specifications for downstream applications. Gas cleanup systems, including particulate removal, tar cracking, sulfur removal, ammonia scrubbing, and moisture removal, are integrated into the plant to purify the syngas.
- Syngas Conditioning: After cleanup, the syngas may undergo additional conditioning processes to adjust its composition and properties for specific end uses. This may include temperature adjustment, gas cooling, gas compression, and gas storage to ensure the syngas meets the requirements of downstream applications.
- Syngas Utilization: The purified syngas is utilized for various energy and chemical production applications, including power generation in gas turbines or internal combustion engines, steam production in boilers, synthesis of liquid fuels (e.g., methanol, Fischer-Tropsch diesel), and production of chemicals (e.g., hydrogen, ammonia, synthetic natural gas).
- Waste Heat Recovery: Biomass gasification plants often generate excess heat during the gasification process, which can be recovered and utilized for district heating, drying biomass feedstocks, or generating additional power through combined heat and power (CHP) systems.
- Emissions Control: Emissions control technologies, such as selective catalytic reduction (SCR), electrostatic precipitators (ESP), and flue gas desulfurization (FGD), are employed to minimize the environmental impact of gasification plant operations and ensure compliance with air quality regulations.
Biomass gasification plants play a vital role in the transition towards sustainable and renewable energy systems by utilizing biomass resources to produce clean and versatile syngas for various energy and industrial applications. Continuous research and development efforts aim to improve the efficiency, reliability, and environmental performance of biomass gasification technologies to enhance their competitiveness and widespread adoption in the renewable energy sector.
Gasification Plant Economics:
Gasification plant economics refer to the financial aspects associated with the development, construction, operation, and maintenance of gasification facilities for the production of syngas from various feedstocks. Understanding the economic factors involved is essential for project feasibility assessment, investment decision-making, and overall project success.
Key considerations in gasification plant economics include:
- Capital Costs: Capital costs encompass the expenses associated with designing, engineering, procuring equipment, constructing, and commissioning the gasification plant. This includes costs for gasification reactors, gas cleanup systems, syngas conditioning equipment, ancillary systems, infrastructure, land acquisition, permitting, and project management. Capital costs are typically the largest component of the total project cost and significantly impact the project’s financial viability.
- Operating Costs: Operating costs include ongoing expenses associated with plant operation and maintenance over its operational lifetime. This includes costs for feedstock procurement, labor, utilities (e.g., electricity, water, fuel), consumables (e.g., catalysts, chemicals), maintenance, repairs, insurance, taxes, and administrative overhead. Operating costs are influenced by factors such as feedstock availability, labor rates, energy prices, regulatory compliance requirements, and plant efficiency.
- Feedstock Costs: Feedstock costs depend on the type, availability, and transportation distance of biomass or other feedstocks used in the gasification process. Biomass feedstock costs may vary based on factors such as feedstock type (e.g., wood chips, agricultural residues, energy crops), quality, seasonal availability, market demand, and competition with other users (e.g., pulp and paper mills, bioenergy plants). The cost of securing a reliable and sustainable feedstock supply is crucial for project economics.
- Syngas Utilization and Revenue Streams: The economic viability of a gasification plant depends on the utilization and monetization of the produced syngas. Revenue streams may include selling syngas for power generation, heat production, biofuels production, chemical synthesis, or supplying syngas to industrial users. The selection of syngas utilization pathways depends on market demand, pricing, infrastructure availability, and regulatory incentives.
- Electricity and Heat Sales: Gasification plants equipped with combined heat and power (CHP) systems can generate electricity and heat simultaneously, increasing overall revenue potential. Selling surplus electricity to the grid or nearby industries can provide additional revenue streams and improve project economics. The economics of electricity sales depend on electricity prices, feed-in tariffs, renewable energy incentives, and grid connection costs.
- Environmental and Regulatory Costs: Gasification plants may incur costs associated with environmental compliance, permitting, emissions monitoring, and regulatory requirements. Compliance with air quality standards, emissions limits, waste disposal regulations, and carbon pricing mechanisms may impose additional costs on plant operations. Implementing emissions control technologies and environmental management practices is essential for minimizing regulatory risks and associated costs.
- Financial Incentives and Subsidies: Government incentives, grants, tax credits, and subsidies can significantly influence the economics of gasification projects. Financial incentives may include investment tax credits, production tax credits, renewable energy certificates (RECs), feed-in tariffs, loan guarantees, and grants for research, development, and demonstration projects. Availability and stability of incentive programs can enhance project economics and attract private investment.
Gasification plant economics require comprehensive financial modeling, risk assessment, sensitivity analysis, and project financing strategies to evaluate investment returns, cash flow projections, and overall project feasibility. Collaborating with financial advisors, lenders, investors, and industry partners can help optimize project economics and secure financing for gasification projects.
Gasification Plant Operations and Maintenance:
Gasification plant operations and maintenance (O&M) encompass the activities involved in running and servicing gasification facilities to ensure optimal performance, reliability, and safety throughout their operational lifespan. Effective O&M practices are essential for maximizing plant efficiency, minimizing downtime, reducing operational costs, and ensuring compliance with regulatory requirements.
Key aspects of gasification plant operations and maintenance include:
- Startup and Shutdown Procedures: Proper startup and shutdown procedures are critical for safely initiating and halting gasification plant operations. During startup, equipment is gradually brought online, and operating parameters are monitored to ensure stable operation. Shutdown procedures involve safely shutting down equipment, purging gas lines, and securing the plant to prevent accidents or equipment damage.
- Process Monitoring and Control: Gasification plant operators continuously monitor process parameters, such as temperature, pressure, flow rates, composition, and energy consumption, to maintain optimal operating conditions and product quality. Advanced process control systems, instrumentation, and automation technologies are employed to optimize gasification performance, adjust process parameters, and respond to variations in feedstock quality or operating conditions.
- Feedstock Handling and Preparation: Proper handling and preparation of biomass feedstocks are crucial for ensuring smooth gasification operations and preventing equipment fouling or damage. Feedstock handling systems, such as conveyors, feeders, and storage silos, are used to transport, store, and meter feedstocks into the gasification reactor. Preprocessing operations, such as drying, size reduction, and screening, may be performed to enhance feedstock characteristics and facilitate gasification.
- Gasification Reactor Maintenance: Gasification reactors require regular inspection, cleaning, and maintenance to ensure efficient and reliable operation. Maintenance activities may include replacing worn or damaged refractory linings, repairing or replacing gasification vessel internals, inspecting and cleaning ash removal systems, and monitoring gasifier performance indicators (e.g., syngas composition, carbon conversion efficiency).
- Gas Cleanup and Conditioning Systems: Gas cleanup and conditioning systems play a crucial role in purifying and conditioning syngas for downstream applications. Maintenance of gas cleanup equipment, such as cyclones, scrubbers, filters, and catalytic converters, involves periodic inspection, cleaning, and replacement of components to maintain optimal performance and minimize emissions.
- Safety and Environmental Compliance: Gasification plant operators must adhere to strict safety protocols, procedures, and regulatory requirements to protect personnel, equipment, and the environment. Safety measures include conducting regular safety training, performing hazard assessments, implementing emergency response plans, and maintaining compliance with occupational health and safety regulations, environmental permits, and emission limits.
- Equipment Reliability and Asset Management: Implementing proactive maintenance strategies, such as predictive maintenance, reliability-centered maintenance (RCM), and condition monitoring, helps optimize equipment reliability, extend asset lifecycles, and minimize unplanned downtime. Regular inspection, lubrication, calibration, and testing of equipment are essential for identifying and addressing potential failures or performance degradation before they escalate into costly disruptions.
Gasification plant operators and maintenance personnel play a critical role in ensuring the safe, efficient, and reliable operation of gasification facilities. Continuous training, skills development, and knowledge transfer are essential for building a competent workforce capable of managing complex gasification processes and maintaining high plant performance standards. Collaboration with equipment suppliers, technology providers, and industry associations can also provide valuable insights and support for optimizing gasification plant operations and maintenance practices.
Gasification Plant Safety Procedures:
Gasification plant safety procedures are essential protocols and practices designed to prevent accidents, mitigate risks, and ensure the safety of personnel, equipment, and the surrounding environment during gasification operations. Adhering to strict safety guidelines and implementing effective safety measures is paramount to protect plant personnel, prevent injuries, and maintain operational integrity.
Key elements of gasification plant safety procedures include:
- Safety Training and Education: Comprehensive safety training programs are provided to all personnel involved in gasification plant operations, including operators, maintenance staff, and contractors. Training covers topics such as hazard recognition, emergency procedures, personal protective equipment (PPE) usage, equipment operation, and safe work practices. Regular refresher training sessions and toolbox talks reinforce safety awareness and promote a safety-oriented culture within the workforce.
- Hazard Identification and Risk Assessment: Gasification plant operators conduct thorough hazard assessments and risk analyses to identify potential hazards, evaluate associated risks, and implement control measures to mitigate risks to an acceptable level. Hazards may include exposure to high temperatures, pressure, toxic gases, flammable materials, electrical hazards, mechanical hazards, and confined spaces. Risk assessments help prioritize safety measures and allocate resources effectively to minimize risk exposure.
- Personal Protective Equipment (PPE): Proper selection and use of personal protective equipment are essential to minimize the risk of injury from workplace hazards. Gasification plant personnel are provided with appropriate PPE, including safety helmets, goggles, face shields, hearing protection, respiratory protection (e.g., respirators, dust masks), gloves, safety footwear, and flame-resistant clothing. PPE usage is mandatory in areas where hazards cannot be adequately controlled through engineering or administrative controls.
- Safe Work Practices: Gasification plant operators follow established safe work practices and standard operating procedures (SOPs) to perform tasks safely and efficiently. This includes procedures for equipment startup and shutdown, lockout/tagout (LOTO) procedures, hot work permits, confined space entry procedures, line breaking procedures, equipment isolation procedures, and emergency response protocols. Compliance with SOPs helps prevent accidents, equipment damage, and environmental incidents.
- Emergency Response Preparedness: Gasification plants maintain comprehensive emergency response plans and procedures to address potential emergencies, such as fires, explosions, gas leaks, chemical spills, and medical emergencies. Emergency response plans include evacuation procedures, assembly points, communication protocols, emergency contact information, firefighting equipment, first aid supplies, and training for emergency responders. Regular drills and exercises are conducted to test emergency response capabilities and improve preparedness.
- Safety Inspections and Audits: Routine safety inspections and audits are conducted to identify potential safety hazards, assess compliance with safety regulations and standards, and implement corrective actions to address deficiencies. Inspections cover equipment integrity, housekeeping practices, electrical safety, fire protection systems, emergency exits, signage, and environmental controls. Management commitment to safety is demonstrated through regular safety audits and proactive measures to address safety concerns.
- Continuous Improvement: Gasification plant safety programs emphasize a culture of continuous improvement, where lessons learned from incidents, near misses, and safety observations are used to enhance safety practices and prevent recurrence. Regular safety meetings, incident investigations, root cause analysis, safety committees, and employee engagement initiatives foster a proactive approach to safety and encourage open communication about safety concerns.
Gasification plant safety procedures are integral to ensuring the well-being of plant personnel, protecting assets, and safeguarding the environment. By prioritizing safety, implementing effective safety measures, and fostering a safety-conscious culture, gasification plants can minimize risks, promote operational excellence, and achieve sustainable long-term success.
Gasification Plant Environmental Management:
Gasification plant environmental management involves implementing strategies, practices, and technologies to minimize the environmental impact of gasification operations and ensure compliance with regulatory requirements. Environmental management efforts focus on reducing emissions, conserving resources, managing waste streams, and promoting sustainable practices to protect air, water, and soil quality.
Key components of gasification plant environmental management include:
- Emission Control Systems: Gasification plants employ advanced emission control systems to capture and treat pollutants generated during gasification processes. This includes the use of particulate matter (PM) removal devices, such as electrostatic precipitators (ESPs), fabric filters, cyclones, and wet scrubbers, to reduce airborne emissions of ash, dust, and other particulates. Additionally, gas cleanup technologies, such as gas cooling and quenching systems, tar removal units, and catalytic converters, are utilized to remove tar, sulfur compounds, and other contaminants from syngas before combustion or further processing.
- Air Quality Monitoring: Gasification plants monitor ambient air quality both within the plant premises and in the surrounding community to assess the impact of plant operations on air quality and ensure compliance with air quality standards and regulations. Continuous emissions monitoring systems (CEMS) are installed to measure and record emissions of criteria pollutants, such as nitrogen oxides (NOx), sulfur dioxide (SO2), carbon monoxide (CO), volatile organic compounds (VOCs), and hazardous air pollutants (HAPs). Real-time data from CEMS are analyzed to identify trends, detect exceedances, and optimize emission control systems for maximum effectiveness.
- Water Management: Gasification plants implement water management strategies to minimize water consumption, prevent water pollution, and protect water resources. This includes implementing water conservation measures, such as recycling and reuse of process water, optimizing cooling water systems, and minimizing wastewater generation through process optimization and containment measures. Effluent treatment facilities are utilized to treat wastewater streams generated during gasification processes before discharge to surface water bodies or municipal treatment facilities. Additionally, stormwater management practices are implemented to prevent runoff and mitigate the risk of contaminant transport into surface water bodies.
- Waste Management: Gasification plants manage waste streams generated during plant operations, including solid waste, ash, and by-products, in accordance with waste management regulations and best practices. Solid waste streams, such as biomass residues, ash, and slag, are segregated, collected, and disposed of or recycled in an environmentally responsible manner. Ash and slag produced during gasification processes may be utilized as construction materials, road aggregate, or soil amendments, reducing the need for landfill disposal. Hazardous wastes, such as spent catalysts or contaminated materials, are handled, stored, and disposed of according to hazardous waste management protocols to prevent environmental contamination.
- Resource Efficiency: Gasification plants strive to optimize resource efficiency and minimize resource consumption through process optimization, energy recovery, and material recycling initiatives. Energy efficiency measures, such as heat integration, cogeneration, and waste heat recovery, are implemented to maximize energy utilization and reduce overall energy consumption. Material recycling and reuse practices are employed to minimize waste generation and conserve natural resources.
- Environmental Compliance: Gasification plants adhere to environmental regulations, permits, and standards governing air emissions, water discharges, waste management, and environmental protection. Compliance monitoring programs are established to track environmental performance, assess regulatory compliance, and report emissions data to regulatory agencies. Environmental audits, inspections, and self-assessments are conducted regularly to identify and address compliance issues, minimize environmental risks, and promote continuous improvement in environmental performance.
Gasification plant environmental management is integral to achieving sustainable operations and minimizing the environmental footprint of gasification processes. By implementing robust environmental management practices, gasification plants can protect the environment, meet regulatory requirements, and enhance their reputation as responsible corporate citizens.
Gasification Plant Maintenance Practices:
Gasification plant maintenance practices encompass a range of activities and procedures aimed at ensuring the reliability, efficiency, and safety of plant equipment and infrastructure. These practices involve preventive maintenance, predictive maintenance, corrective maintenance, and proactive maintenance strategies to address equipment degradation, mitigate failures, and optimize plant performance.
Key elements of gasification plant maintenance practices include:
- Preventive Maintenance: Preventive maintenance involves scheduled inspections, servicing, and repairs of equipment and systems to prevent failures, extend equipment life, and maintain operational reliability. This includes routine tasks such as equipment lubrication, filter replacements, valve inspections, and equipment calibration. Preventive maintenance schedules are developed based on equipment manufacturer recommendations, operating experience, and reliability-centered maintenance (RCM) principles to identify critical maintenance tasks and prioritize maintenance activities.
- Predictive Maintenance: Predictive maintenance techniques are utilized to monitor equipment condition, detect early signs of deterioration, and predict potential failures before they occur. This includes the use of condition monitoring technologies such as vibration analysis, thermography, oil analysis, ultrasonic testing, and online monitoring systems to assess equipment health and identify abnormalities indicative of impending failures. Predictive maintenance allows maintenance activities to be scheduled based on actual equipment condition, minimizing downtime and reducing maintenance costs.
- Corrective Maintenance: Corrective maintenance involves responding to equipment failures, malfunctions, or abnormalities and restoring equipment to its operational condition in a timely manner. This includes troubleshooting, diagnosis, repair, and replacement of faulty components or systems to minimize downtime and resume normal operations. Corrective maintenance may be planned or unplanned, depending on the nature of the failure and its impact on plant performance.
- Proactive Maintenance: Proactive maintenance focuses on identifying and addressing root causes of equipment degradation or failures to prevent recurrence and improve overall reliability. This includes implementing reliability improvement initiatives, implementing design upgrades, optimizing maintenance practices, and addressing chronic maintenance issues through root cause analysis (RCA) and failure mode and effects analysis (FMEA). Proactive maintenance aims to eliminate repetitive failures, enhance equipment performance, and optimize maintenance strategies to achieve higher levels of reliability and availability.
- Asset Management: Gasification plants implement asset management programs to optimize the lifecycle management of plant assets, including equipment, infrastructure, and spare parts. This includes asset tracking, inventory management, obsolescence management, and strategic planning for asset replacement or refurbishment. Asset management practices help optimize capital investments, minimize lifecycle costs, and ensure the availability of critical spare parts and components to support maintenance activities.
- Training and Skills Development: Gasification plant personnel receive training and skills development opportunities to enhance their technical competencies, knowledge of equipment, and maintenance best practices. This includes training on equipment operation, maintenance procedures, safety protocols, and emerging technologies. Continuous learning and skills development programs ensure that maintenance staff are equipped with the necessary skills and knowledge to perform their roles effectively and adapt to changing maintenance requirements.
Gasification plant maintenance practices are essential for ensuring the reliable and efficient operation of plant assets, minimizing downtime, and optimizing plant performance. By implementing proactive maintenance strategies, leveraging predictive maintenance technologies, and investing in training and skills development, gasification plants can achieve higher levels of reliability, availability, and operational excellence.
Gasification Plant Safety Protocols:
Gasification plant safety protocols encompass a comprehensive set of measures, procedures, and practices designed to protect personnel, assets, and the environment from potential hazards associated with gasification processes. Safety protocols are implemented to prevent accidents, minimize risks, and ensure compliance with regulatory requirements and industry standards.
Key components of gasification plant safety protocols include:
- Risk Assessment: Gasification plants conduct thorough risk assessments to identify and evaluate potential hazards associated with plant operations, equipment, materials, and processes. This includes hazard identification, risk analysis, and risk mitigation planning to identify high-risk areas, prioritize safety interventions, and implement appropriate control measures to mitigate risks.
- Safety Training: Gasification plant personnel receive comprehensive safety training to familiarize them with safety protocols, procedures, and emergency response plans. This includes training on hazard awareness, chemical safety, personal protective equipment (PPE) usage, emergency procedures, fire safety, confined space entry, and equipment operation. Safety training programs are tailored to specific job roles and responsibilities to ensure that personnel are equipped with the knowledge and skills to work safely and respond effectively to emergencies.
- Personal Protective Equipment (PPE): Gasification plant personnel are required to wear appropriate PPE to protect against occupational hazards and risks. This includes protective clothing, safety helmets, safety glasses, hearing protection, respiratory protection, and fall protection equipment as necessary. PPE usage is enforced in areas where hazards cannot be eliminated or adequately controlled through engineering or administrative controls.
- Process Safety Management (PSM): Gasification plants implement process safety management systems to identify, assess, and control process-related hazards and risks. This includes implementing process safety information (PSI) systems, process hazard analysis (PHA), operating procedures, mechanical integrity programs, management of change (MOC) procedures, emergency response plans, and incident investigation protocols to prevent process-related accidents and protect plant personnel, assets, and the environment.
- Emergency Preparedness and Response: Gasification plants develop and maintain comprehensive emergency preparedness and response plans to address potential emergencies, including fires, explosions, chemical releases, and natural disasters. This includes establishing emergency response teams, conducting drills and exercises, coordinating with local emergency responders, and providing emergency response training to plant personnel. Emergency response plans are regularly reviewed, updated, and tested to ensure readiness and effectiveness in managing emergency situations.
- Safety Inspections and Audits: Gasification plants conduct regular safety inspections, audits, and assessments to identify safety hazards, assess compliance with safety regulations and standards, and identify opportunities for improvement. This includes conducting site inspections, safety walkthroughs, equipment inspections, and safety audits by qualified personnel to identify safety deficiencies, hazards, and non-compliance issues. Corrective actions are implemented promptly to address identified deficiencies and improve safety performance.
- Continuous Improvement: Gasification plants promote a culture of continuous improvement in safety performance by encouraging employee participation, feedback, and suggestions for safety enhancements. This includes establishing safety committees, conducting safety meetings, and implementing safety recognition programs to recognize and reward safety excellence. Regular safety performance reviews are conducted to monitor progress, identify trends, and implement corrective actions to continuously improve safety performance.
Gasification plant safety protocols are essential for protecting personnel, assets, and the environment from potential hazards associated with gasification processes. By implementing comprehensive safety measures, providing effective training, and fostering a culture of safety excellence, gasification plants can minimize risks, prevent accidents, and ensure the safe and reliable operation of plant facilities.
Gasification Plant Environmental Management:
Gasification plant environmental management refers to the comprehensive set of practices, policies, and procedures implemented to minimize the environmental impact of plant operations, mitigate emissions, and ensure compliance with environmental regulations and standards. Environmental management aims to protect air quality, water resources, soil integrity, and biodiversity while promoting sustainable and responsible plant operations.
Key components of gasification plant environmental management include:
- Environmental Impact Assessment (EIA): Gasification plants conduct environmental impact assessments to evaluate the potential environmental effects of plant construction, operation, and decommissioning. EIAs assess the potential impacts on air quality, water resources, soil quality, biodiversity, and human health to identify mitigation measures and ensure compliance with environmental regulations.
- Emissions Monitoring and Control: Gasification plants implement emissions monitoring and control systems to measure, monitor, and control air emissions generated during plant operations. This includes monitoring emissions of criteria pollutants such as particulate matter (PM), nitrogen oxides (NOx), sulfur dioxide (SO2), carbon monoxide (CO), volatile organic compounds (VOCs), and hazardous air pollutants (HAPs). Emissions control technologies such as electrostatic precipitators, scrubbers, catalytic converters, and selective catalytic reduction (SCR) systems are utilized to minimize emissions and comply with regulatory limits.
- Waste Management: Gasification plants manage waste generated during plant operations, including solid waste, hazardous waste, and wastewater, in accordance with applicable regulations and standards. This includes implementing waste minimization measures, recycling and reuse programs, proper storage and handling practices, and disposal methods such as incineration, landfilling, or recycling. Waste management practices aim to minimize environmental impacts, reduce pollution, and promote resource conservation.
- Water Management: Gasification plants implement water management practices to minimize water consumption, protect water quality, and manage wastewater generated during plant operations. This includes implementing water conservation measures, recycling and reuse of water where feasible, and treating wastewater to remove contaminants before discharge. Water management practices aim to protect surface water and groundwater resources, prevent water pollution, and ensure compliance with water quality regulations.
- Land Use and Site Remediation: Gasification plants manage land use and implement site remediation measures to minimize the impact of plant operations on land resources and ecosystems. This includes implementing erosion and sediment control measures, revegetation programs, and soil stabilization techniques to minimize soil erosion and preserve soil integrity. Site remediation may be necessary to address soil and groundwater contamination resulting from historical industrial activities and to restore impacted areas to their natural state.
- Environmental Compliance: Gasification plants maintain compliance with environmental regulations and standards through ongoing monitoring, reporting, and verification of environmental performance. This includes obtaining permits and approvals from regulatory agencies, conducting environmental audits and inspections, and submitting regulatory reports on air emissions, wastewater discharges, and hazardous waste management. Environmental compliance ensures that plant operations are conducted in a manner that protects human health and the environment while minimizing regulatory risks.
Gasification plant environmental management practices are essential for minimizing the environmental footprint of plant operations, protecting natural resources, and promoting sustainable development. By implementing comprehensive environmental management programs, gasification plants can minimize environmental impacts, comply with regulatory requirements, and demonstrate environmental stewardship and responsibility.
Gasification Plant Process Optimization:
Gasification plant process optimization involves the systematic analysis, improvement, and fine-tuning of gasification processes to enhance efficiency, productivity, and performance while minimizing costs and environmental impacts. Optimization efforts focus on maximizing the conversion of feedstock into syngas or other valuable products, improving energy efficiency, reducing emissions, and ensuring the overall reliability and operability of the gasification plant.
Key aspects of gasification plant process optimization include:
- Feedstock Selection and Preparation: Optimal feedstock selection is critical for maximizing gasification performance. Process optimization involves evaluating different feedstock options based on availability, cost, energy content, and suitability for gasification. Additionally, feedstock preparation techniques such as drying, size reduction, and sorting are optimized to ensure consistent feed quality and optimal process performance.
- Gasifier Design and Operation: Gasifier design and operation play a crucial role in gasification plant performance. Optimization efforts focus on selecting the appropriate gasifier type (e.g., fixed-bed, fluidized bed, entrained flow) and optimizing operating parameters such as temperature, pressure, residence time, and feedstock-to-air ratio to maximize gasification efficiency and syngas quality while minimizing tar formation and other undesired by-products.
- Syngas Cleanup and Conditioning: Syngas produced during gasification typically contains impurities such as tar, particulates, sulfur compounds, and nitrogen oxides (NOx). Optimization of syngas cleanup and conditioning processes involves selecting and optimizing appropriate gas cleaning technologies such as scrubbers, filters, catalytic converters, and adsorption systems to remove impurities and contaminants from the syngas stream, ensuring compliance with product specifications and environmental regulations.
- Syngas Utilization and Product Recovery: Optimizing syngas utilization and product recovery processes is essential for maximizing the value of gasification products. This includes optimizing the operation of downstream processes such as syngas upgrading, syngas-to-liquids (STL) conversion, hydrogen production, power generation, and chemical synthesis to maximize product yields, quality, and profitability.
- Energy Integration and Heat Recovery: Gasification plant process optimization includes maximizing energy efficiency and heat recovery throughout the plant. This involves integrating heat exchangers, heat recovery steam generators (HRSGs), and other energy recovery systems to capture and utilize waste heat for process heating, steam generation, and power generation, thereby reducing energy consumption and operating costs.
- Control and Automation Systems: Optimization of control and automation systems plays a crucial role in gasification plant operation. Advanced process control (APC) strategies, predictive modeling, and real-time optimization techniques are employed to optimize process parameters, enhance process stability, and minimize off-spec production. Additionally, remote monitoring and diagnostic systems are utilized to identify performance bottlenecks, troubleshoot issues, and implement corrective actions in real time.
- Data Analytics and Performance Monitoring: Gasification plant optimization relies on data analytics and performance monitoring to identify opportunities for improvement and track key performance indicators (KPIs). Advanced data analytics techniques such as machine learning, artificial intelligence (AI), and predictive analytics are employed to analyze process data, identify trends, and predict equipment failures or process deviations before they occur, enabling proactive maintenance and optimization.
Gasification plant process optimization is an ongoing endeavor that requires collaboration between process engineers, plant operators, and automation experts to continuously identify opportunities for improvement, implement optimization strategies, and maximize the overall efficiency, productivity, and profitability of gasification operations.
Pyrolysis
- Pyrolysis: Pyrolysis is a thermochemical decomposition process that involves heating biomass or organic materials in the absence of oxygen to produce biochar, bio-oil, and syngas. During pyrolysis, biomass is subjected to high temperatures (typically between 300°C and 800°C) in an oxygen-limited environment, leading to the breakdown of complex organic molecules into simpler compounds.
- Biochar: Biochar is a carbon-rich solid material produced from the pyrolysis of biomass. It is a highly porous substance with a large surface area, which makes it suitable for various applications, including soil amendment, carbon sequestration, and environmental remediation. Biochar can improve soil fertility, water retention, and nutrient retention, making it a valuable product for sustainable agriculture and land management practices.
- Bio-oil: Bio-oil, also known as pyrolysis oil or bio-crude, is a liquid product obtained from the pyrolysis of biomass. It is a complex mixture of organic compounds, including hydrocarbons, oxygenates, and water. Bio-oil has potential applications as a renewable fuel for heat and power generation, as well as a feedstock for the production of bio-based chemicals and transportation fuels through upgrading processes such as hydrotreating and hydrodeoxygenation.
- Syngas: Syngas, or synthesis gas, is a mixture of hydrogen (H2), carbon monoxide (CO), and other trace gases produced from the pyrolysis of biomass or other carbonaceous materials. Syngas can be used as a fuel for combustion processes, such as heating and power generation, or as a feedstock for the production of synthetic fuels, chemicals, and hydrogen.
- Biomass Pyrolysis: Biomass pyrolysis is the process of converting biomass into biochar, bio-oil, and syngas through thermal decomposition in the absence of oxygen. It is a promising technology for converting renewable biomass resources into valuable bioenergy and bio-based products, with potential applications in waste management, energy production, and carbon sequestration.
- Fast Pyrolysis: Fast pyrolysis is a rapid heating process that involves heating biomass at high temperatures (typically above 400°C) for a short residence time (usually a few seconds to minutes) to produce bio-oil, biochar, and syngas. Fast pyrolysis technologies enable the efficient conversion of biomass into liquid and gaseous fuels with higher yields and faster reaction rates compared to slow pyrolysis processes.
- Slow Pyrolysis: Slow pyrolysis is a slow heating process that involves heating biomass at lower temperatures (typically between 300°C and 500°C) for a longer residence time (hours to days) to produce biochar, bio-oil, and syngas. Slow pyrolysis technologies are characterized by slower heating rates and longer reaction times, resulting in higher biochar yields and lower bio-oil production compared to fast pyrolysis processes.
- Flash Pyrolysis: Flash pyrolysis is an ultra-fast heating process that involves rapidly heating biomass at very high temperatures (above 800°C) for an extremely short residence time (milliseconds to seconds) to produce mainly bio-oil and syngas. Flash pyrolysis technologies utilize high-temperature, high-velocity reactors or entrained flow reactors to achieve rapid heating rates and maximize bio-oil yields.
- Catalytic Pyrolysis: Catalytic pyrolysis is a pyrolysis process that involves the use of catalysts to enhance the yield and quality of pyrolysis products, such as bio-oil and syngas. Catalysts promote the cracking, deoxygenation, and reforming reactions during pyrolysis, leading to higher bio-oil yields, lower oxygen content, and improved stability of the resulting products. Catalysts commonly used in catalytic pyrolysis include zeolites, metal oxides, and supported metal catalysts.
- Bioenergy: Bioenergy refers to energy derived from biomass resources, including organic materials such as wood, agricultural residues, and energy crops. Pyrolysis is a bioenergy conversion technology that enables the production of biofuels, biochar, and biogas from biomass feedstocks, offering renewable and sustainable alternatives to fossil fuels for heat, power, and transportation applications.
- Biofuel: Biofuels are liquid or gaseous fuels derived from biomass resources, including bio-oil produced through pyrolysis. Biofuels can be used as renewable alternatives to fossil fuels for transportation, heating, and power generation, offering lower greenhouse gas emissions and reduced dependence on finite fossil fuel resources.
- Carbon Sequestration: Carbon sequestration is the process of capturing and storing carbon dioxide (CO2) from the atmosphere to mitigate climate change and reduce greenhouse gas emissions. Biochar produced through biomass pyrolysis can act as a long-term carbon sink when applied to soils, helping to sequester carbon and improve soil fertility and resilience to climate change.
- Renewable Energy: Renewable energy refers to energy derived from naturally replenishable sources, such as biomass, sunlight, wind, and water. Pyrolysis technologies enable the conversion of biomass into renewable energy products, including bio-oil, biochar, and syngas, which can be used to generate heat, electricity, and transportation fuels without depleting finite fossil fuel reserves or emitting greenhouse gases.
- Bio-based Chemicals: Bio-based chemicals are chemicals derived from biomass feedstocks rather than fossil fuels. Pyrolysis can be used to produce bio-oil, which can be further upgraded and processed into a wide range of bio-based chemicals, such as bio-based plastics, solvents, and lubricants, offering sustainable alternatives to petrochemical-derived products.
- Thermal Conversion: Thermal conversion is a group of technologies that involve the use of heat to convert biomass into energy and other valuable products. Pyrolysis is one of the thermal conversion processes used to decompose biomass into bio-oil, biochar, and syngas through heating in the absence of oxygen, offering an efficient and environmentally friendly approach to biomass utilization.
- Hydrothermal Pyrolysis: Hydrothermal pyrolysis is a pyrolysis process that involves heating wet biomass in the presence of water at elevated temperatures and pressures to produce bio-oil, biochar, and gases. Hydrothermal pyrolysis technologies utilize supercritical water or subcritical water conditions to enhance the conversion of biomass into liquid and gaseous fuels while minimizing the formation of char and tar.
- Gasification: Gasification is a thermochemical conversion process that involves the partial oxidation of biomass or other carbonaceous materials to produce syngas, a mixture of hydrogen, carbon monoxide, and other gases. Although gasification is distinct from pyrolysis, both processes are often used in combination to convert biomass into valuable energy products, such as biofuels and chemicals.
- Carbonization: Carbonization is the process of converting biomass into carbon-rich materials such as charcoal or activated carbon through heating in the absence of oxygen. Although carbonization is similar to pyrolysis, it typically involves slower heating rates and higher temperatures, resulting in the production of char-based products with specific properties for various applications, including filtration, adsorption, and metallurgy.
- Biomass Valorization: Biomass valorization refers to the conversion of biomass into high-value products, including energy, chemicals, and materials, through various conversion processes such as pyrolysis, gasification, and fermentation. Pyrolysis plays a key role in biomass valorization by enabling the production of bio-based fuels and chemicals from renewable biomass resources, contributing to the transition to a sustainable bioeconomy.
- Waste-to-Energy: Waste-to-energy (WtE) refers to the generation of energy from waste materials through thermal, biological, or chemical conversion processes. Pyrolysis is a waste-to-energy technology that can convert organic waste materials, such as agricultural residues, municipal solid waste, and sewage sludge, into bioenergy products, reducing waste disposal costs, minimizing environmental pollution, and promoting resource recovery and circular economy principles.
Pyrolysis:
Pyrolysis is a thermal decomposition process that involves the heating of biomass in the absence of oxygen to produce biochar, bio-oil, and syngas. During pyrolysis, biomass undergoes rapid heating to temperatures typically ranging from 300°C to 800°C, causing its chemical bonds to break down and release volatile compounds. These volatile compounds are then condensed into bio-oil, while the remaining solid residue is converted into biochar. The syngas produced during pyrolysis consists mainly of hydrogen, carbon monoxide, and methane and can be further processed or utilized for energy production.
Pyrolysis can be categorized into three main types: slow pyrolysis, fast pyrolysis, and flash pyrolysis. Slow pyrolysis involves heating biomass at lower temperatures (300°C to 500°C) over an extended period, resulting in higher yields of biochar and lower yields of bio-oil. Fast pyrolysis, on the other hand, employs higher heating rates (greater than 100°C/s) and shorter residence times (typically less than 2 seconds), leading to higher bio-oil yields and lower biochar yields. Flash pyrolysis involves even faster heating rates and shorter residence times, often using hot sand or fluidized bed reactors to achieve rapid biomass decomposition.
Pyrolysis offers several advantages as a biomass conversion technology. It can process a wide range of feedstocks, including forestry residues, agricultural residues, energy crops, and municipal solid waste, making it versatile for various biomass sources. The produced biochar can be used as a soil amendment to improve soil fertility, carbon sequestration, and water retention. Bio-oil obtained from pyrolysis can be further upgraded to produce transportation fuels, specialty chemicals, and other value-added products.
However, pyrolysis also faces certain challenges, such as high energy consumption during biomass drying and heating, complex product compositions requiring downstream processing for separation and purification, and potential environmental impacts from emissions and waste management. Research and development efforts continue to focus on optimizing pyrolysis processes, improving product yields and quality, and reducing environmental footprints to enhance the economic and environmental sustainability of pyrolysis-based bioenergy systems.
Gasification:
Gasification is a thermochemical conversion process that involves the partial oxidation of biomass or other carbonaceous materials to produce syngas, a mixture of hydrogen, carbon monoxide, and other gases. The process takes place in a gasifier, where biomass is heated in the presence of a controlled amount of oxygen, steam, or both, at high temperatures (typically 700°C to 1,500°C) and elevated pressures.
During gasification, biomass undergoes several chemical reactions, including pyrolysis, combustion, and gas-phase reactions. Pyrolysis occurs first, breaking down the complex organic compounds in biomass into volatile gases, tars, and char. The volatile gases then react with oxygen or steam to undergo further conversion reactions, primarily producing syngas composed of hydrogen (H2) and carbon monoxide (CO). The resulting syngas can be used as a fuel for heat and power generation, or as a feedstock for the production of biofuels, chemicals, and other value-added products.
Gasification offers several advantages over traditional combustion processes. It allows for the efficient utilization of biomass feedstocks, including forestry residues, agricultural wastes, energy crops, and municipal solid waste, by converting them into a versatile gaseous fuel. Syngas produced from gasification has a higher energy content and can be more easily stored, transported, and utilized compared to solid biomass. Gasification also enables the integration of renewable biomass resources with existing energy infrastructure, such as power plants and industrial facilities, to reduce greenhouse gas emissions and promote energy security and sustainability.
However, gasification also presents certain challenges and considerations. The process requires careful control of operating parameters, such as temperature, pressure, and residence time, to optimize syngas composition and minimize undesirable by-products, such as tars and particulates. Gasification systems can be complex and capital-intensive, requiring significant investment in equipment and infrastructure. Additionally, the quality and availability of biomass feedstocks, as well as regulatory and policy frameworks, can influence the economic viability and scalability of gasification projects.
Research and development efforts in gasification technology continue to focus on improving process efficiency, reliability, and flexibility, as well as exploring novel feedstock options and integrated biorefinery concepts. By addressing these challenges and advancing gasification technology, it holds significant promise for advancing the transition to a sustainable, low-carbon energy future.
Carbonization:
Carbonization is a thermochemical conversion process that involves heating biomass in the absence of oxygen to produce carbon-rich materials such as charcoal, biochar, or activated carbon. Unlike combustion or gasification, carbonization occurs under limited oxygen conditions, preventing complete combustion and allowing carbonaceous residues to accumulate.
The carbonization process typically takes place in a carbonization reactor, such as a kiln or retort, where biomass feedstock is heated to temperatures ranging from 300°C to 900°C. At these elevated temperatures, biomass undergoes thermal decomposition, releasing volatile organic compounds, water vapor, and other gases. As oxygen is absent, these gases are not combusted but are instead expelled from the reactor.
The remaining solid residue, known as char or carbonized biomass, consists primarily of carbon and ash and retains the structure and morphology of the original biomass feedstock. The properties of the resulting char depend on various factors, including the type of biomass, heating rate, temperature, and residence time. For example, slow carbonization at lower temperatures tends to produce biochar with higher porosity and surface area, making it suitable for applications such as soil amendment, carbon sequestration, and water filtration.
In addition to biochar, carbonization processes can also yield other carbonaceous materials with specific properties and applications. Charcoal, for instance, is a carbonized biomass commonly used as a cooking fuel, industrial reductant, or raw material for activated carbon production. Activated carbon, produced by further processing char with steam or chemicals, exhibits high porosity and surface area, making it valuable for adsorption, purification, and catalysis in various industrial, environmental, and medical applications.
Carbonization offers several advantages as a biomass conversion technology. It can convert a wide range of biomass feedstocks, including wood, agricultural residues, and organic waste, into stable, carbon-rich materials with useful properties. The resulting biochar can improve soil fertility, enhance carbon sequestration, and mitigate greenhouse gas emissions when applied to agricultural or forestry lands. Furthermore, charcoal and activated carbon derived from carbonization can substitute for fossil-based materials in various industrial processes, contributing to resource efficiency and environmental sustainability.
However, carbonization also presents certain challenges and considerations. The process requires careful control of operating parameters to ensure the desired product quality and yield, as well as efficient energy utilization. Additionally, the economics of carbonization can vary depending on factors such as feedstock availability, processing costs, and market demand for end products. Continued research and development efforts aim to optimize carbonization processes, develop value-added applications for carbonaceous materials, and enhance the overall sustainability of biomass carbonization technologies.
Syngas:
Syngas, short for synthesis gas, is a versatile mixture of gases primarily composed of hydrogen (H2) and carbon monoxide (CO), along with other trace gases such as carbon dioxide (CO2), methane (CH4), and nitrogen (N2). It is produced through the thermochemical conversion of carbon-containing feedstocks, such as biomass, coal, or natural gas, in processes such as gasification or steam reforming.
The composition of syngas can vary depending on the feedstock and the specific conditions of the conversion process. In biomass gasification, for example, syngas is typically produced by reacting biomass with a controlled amount of oxygen (O2), steam (H2O), or a combination of both at elevated temperatures (typically between 700°C and 1,500°C) in the absence of air. This thermochemical reaction breaks down the complex organic compounds present in biomass into simpler molecules, primarily hydrogen and carbon monoxide, through a series of chemical reactions including pyrolysis, oxidation, and reduction.
Syngas has a wide range of applications across various industries due to its combustible nature and chemical versatility. It can be used as a fuel for heat and power generation in gas turbines, internal combustion engines, or fuel cells, providing a renewable and cleaner alternative to fossil fuels. Syngas can also serve as a feedstock for the production of a wide range of valuable chemicals and fuels through processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia synthesis. Additionally, syngas can be further processed to remove impurities such as sulfur compounds and particulates before utilization or storage.
One of the key advantages of syngas is its flexibility and compatibility with existing infrastructure, making it a promising candidate for the transition to a low-carbon energy future. By utilizing renewable feedstocks such as biomass or waste materials, syngas production can help reduce greenhouse gas emissions and dependence on fossil fuels while promoting energy security and sustainability. However, challenges such as process efficiency, feedstock availability, and downstream processing remain areas of ongoing research and development to further optimize syngas production and utilization technologies.
Biomass Gasification:
Biomass gasification is a thermochemical process that converts biomass feedstocks into a gaseous fuel called syngas, or synthesis gas. This process occurs in a gasifier, where biomass is subjected to high temperatures and controlled amounts of oxygen (or air) and/or steam in a limited-oxygen environment. The biomass undergoes several sequential reactions, including drying, pyrolysis, oxidation, and reduction, resulting in the production of syngas.
The gasification process begins with the drying of the biomass, during which moisture is removed from the feedstock. Subsequently, as the temperature increases, pyrolysis occurs, breaking down the complex organic molecules in the biomass into volatile gases, tars, and char. These volatile gases then undergo further reactions in the presence of oxygen or steam. In the presence of oxygen, partial combustion reactions occur, producing carbon dioxide (CO2) and water vapor (H2O). In contrast, when steam is introduced, the volatile gases undergo the water-gas shift reaction, producing additional hydrogen (H2) and carbon monoxide (CO) while reducing the formation of carbon dioxide.
The resulting syngas is a mixture of hydrogen, carbon monoxide, carbon dioxide, methane, and trace amounts of other gases. The composition of syngas can be adjusted by controlling the operating parameters of the gasifier, such as temperature, pressure, residence time, and feedstock composition. After gasification, the syngas can be cleaned and conditioned to remove impurities such as tars, particulates, and sulfur compounds before being utilized as a fuel for various applications.
Biomass gasification offers several advantages over conventional combustion technologies. It enables the efficient utilization of diverse biomass feedstocks, including agricultural residues, forestry wastes, energy crops, and organic waste materials, by converting them into a versatile gaseous fuel. Syngas produced from biomass gasification has a high energy content and can be used for combined heat and power (CHP) generation, industrial processes, and transportation fuels. Additionally, biomass gasification can help reduce greenhouse gas emissions, promote energy security, and mitigate environmental pollution associated with conventional fossil fuel use.
However, biomass gasification also faces challenges and limitations. The process requires careful control of operating conditions to optimize syngas quality and minimize the formation of undesirable by-products such as tars and particulates. The economics of biomass gasification are influenced by factors such as feedstock availability, capital costs, energy prices, and policy incentives. Continued research and development efforts aim to improve the efficiency, reliability, and scalability of biomass gasification technologies to accelerate the transition to a sustainable and renewable energy future.
Gasifier:
A gasifier is a device used in the thermochemical conversion of solid carbonaceous materials, such as biomass, coal, or waste, into a combustible gas mixture known as syngas. This process, called gasification, occurs in a gasifier reactor where the solid feedstock is subjected to high temperatures and controlled amounts of oxygen, steam, or a combination of both in a limited-oxygen environment.
Gasifiers come in various designs and configurations, but they generally consist of a reaction chamber where the feedstock undergoes several sequential thermochemical reactions. These reactions include drying, pyrolysis, oxidation, and reduction, leading to the formation of syngas. The gasifier may also include additional features such as feedstock feeding systems, heat exchange surfaces, and gas cleaning units to improve efficiency and control emissions.
In the gasification process, the solid feedstock is first dried to remove moisture content, followed by pyrolysis, where the biomass is heated in the absence of oxygen, leading to the release of volatile organic compounds, tars, and char. Subsequently, the volatile gases undergo further reactions in the presence of oxygen or steam. Depending on the operating conditions and feedstock composition, these reactions can result in the production of syngas composed primarily of hydrogen (H2) and carbon monoxide (CO), along with other gases such as carbon dioxide (CO2), methane (CH4), and nitrogen (N2).
Gasifiers can be classified based on their operating principles, feedstock types, and reactor configurations. Common types of gasifiers include fixed-bed gasifiers, fluidized-bed gasifiers, entrained-flow gasifiers, and downdraft gasifiers, each offering different advantages and limitations in terms of efficiency, feedstock flexibility, and syngas quality.
Gasification offers several advantages over conventional combustion technologies. It enables the efficient utilization of a wide range of feedstocks, including biomass residues, agricultural wastes, and municipal solid waste, by converting them into a clean and versatile syngas fuel. Syngas produced from gasification can be used for various applications, including heat and power generation, industrial processes, and synthesis of fuels and chemicals, contributing to energy security, environmental sustainability, and resource efficiency.
However, gasification also presents challenges and considerations, such as the need for careful control of operating parameters, gas cleaning requirements, and capital costs. Ongoing research and development efforts aim to advance gasification technologies, optimize process efficiency, and expand the range of feedstocks and applications to accelerate the transition to a sustainable and low-carbon energy future.
Thermal Conversion:
Thermal conversion refers to a group of processes that utilize heat to transform various types of feedstocks into useful products, including energy, fuels, and chemicals. These processes rely on the application of heat to drive chemical reactions and physical transformations, typically occurring at elevated temperatures and often in the absence of oxygen or with controlled amounts of oxygen.
One of the key thermal conversion processes is pyrolysis, which involves the decomposition of organic materials, such as biomass, plastics, or waste, into a mixture of gases, liquids, and solids in the absence of oxygen. During pyrolysis, the feedstock is heated to temperatures ranging from a few hundred to over a thousand degrees Celsius, causing it to undergo thermal decomposition, releasing volatile compounds and leaving behind char or solid residues. The resulting products can be further processed and refined to obtain valuable fuels, such as bio-oil, syngas, and biochar, or used directly for heat and power generation.
Another thermal conversion process is gasification, which converts carbonaceous materials, such as biomass, coal, or waste, into a combustible gas mixture known as syngas. Gasification involves the partial oxidation of the feedstock at high temperatures (typically above 700°C) in a controlled environment with a limited supply of oxygen or steam. The resulting syngas can be used as a clean and versatile fuel for heat and power generation, as well as for the production of chemicals and transportation fuels.
Thermal conversion also includes processes such as combustion, where organic materials are oxidized in the presence of oxygen to release heat energy; and liquefaction, which converts solid or gaseous feedstocks into liquid fuels through the application of heat and pressure.
The choice of thermal conversion process depends on factors such as the feedstock characteristics, desired end products, and process efficiency. Thermal conversion technologies offer several advantages, including the ability to utilize a wide range of feedstocks, generate renewable energy, and reduce waste and greenhouse gas emissions. However, challenges such as process optimization, feedstock availability, and economic viability need to be addressed to fully realize the potential of thermal conversion for sustainable energy production and resource utilization. Ongoing research and development efforts are focused on improving the efficiency, reliability, and environmental performance of thermal conversion technologies to meet the growing demand for clean and renewable energy sources.
Renewable Energy:
Renewable energy refers to energy derived from natural resources that are continuously replenished and can be utilized without depleting finite resources or causing significant environmental harm. Unlike fossil fuels, which are non-renewable and finite, renewable energy sources are abundant and sustainable over the long term, making them crucial components of a transition towards a more sustainable and low-carbon energy future.
There are various types of renewable energy sources, each with its own unique characteristics and applications. These include:
- Solar Energy: Solar energy harnesses the power of sunlight to generate electricity through photovoltaic (PV) panels or to produce heat through solar thermal systems. Solar power is abundant, widely distributed, and can be harnessed both at large-scale utility installations and on rooftops of buildings for distributed generation.
- Wind Energy: Wind energy utilizes the kinetic energy of wind to drive turbines and generate electricity. Wind power is a mature and rapidly growing renewable energy technology, with large-scale wind farms deployed onshore and offshore in areas with favorable wind conditions.
- Hydropower: Hydropower captures the energy of flowing water, such as rivers or waterfalls, to generate electricity. Hydropower plants can range from large-scale dams and reservoirs to small-scale run-of-river installations, providing a reliable and flexible source of renewable energy.
- Biomass Energy: Biomass energy utilizes organic materials, such as wood, agricultural residues, and organic waste, to generate heat, electricity, or biofuels through processes such as combustion, gasification, and anaerobic digestion. Biomass energy can help reduce greenhouse gas emissions and provide a renewable alternative to fossil fuels.
- Geothermal Energy: Geothermal energy taps into heat stored beneath the Earth’s surface to generate electricity or provide direct heating and cooling. Geothermal power plants utilize hot water or steam from geothermal reservoirs to drive turbines and produce electricity.
- Ocean Energy: Ocean energy encompasses various technologies that harness the energy of the ocean, including tidal energy, wave energy, and ocean thermal energy conversion (OTEC). These technologies utilize the kinetic energy of tides and waves or the temperature difference between surface and deep ocean waters to generate electricity.
Renewable energy sources offer numerous environmental, economic, and social benefits compared to fossil fuels, including reduced greenhouse gas emissions, energy security, job creation, and local economic development. However, challenges such as intermittency, grid integration, and resource availability need to be addressed to maximize the potential of renewable energy and accelerate the transition to a sustainable and resilient energy system. Continued research, innovation, and policy support are essential to overcome these challenges and achieve a more sustainable energy future powered by renewable sources.
Carbon Capture and Storage (CCS):
Carbon capture and storage (CCS) is a process designed to mitigate greenhouse gas emissions, particularly carbon dioxide (CO2), from large-scale point sources such as power plants and industrial facilities. CCS involves capturing CO2 emissions produced from the combustion of fossil fuels or other industrial processes, transporting the captured CO2 to a suitable storage site, and securely storing it underground to prevent its release into the atmosphere.
The CCS process typically consists of three main steps: capture, transportation, and storage.
- Capture: CO2 capture technologies capture CO2 emissions from industrial processes before they are released into the atmosphere. There are various capture technologies available, including post-combustion capture, pre-combustion capture, and oxy-fuel combustion. Post-combustion capture involves removing CO2 from the flue gases emitted by power plants or industrial facilities using chemical solvents or sorbents. Pre-combustion capture involves converting fossil fuels into syngas (a mixture of hydrogen and carbon monoxide) before combustion, allowing for easier separation of CO2. Oxy-fuel combustion involves burning fossil fuels in oxygen-rich environments to produce flue gases with high concentrations of CO2.
- Transportation: Once captured, CO2 is transported via pipelines, ships, or trucks to suitable storage sites. Pipelines are the most common method of transportation for large-scale CCS projects, as they provide a cost-effective and efficient means of transporting CO2 over long distances. Ships and trucks are used for smaller-scale CCS projects or for transporting CO2 to remote storage sites.
- Storage: CO2 is injected deep underground into geological formations, such as depleted oil and gas reservoirs, saline aquifers, or deep coal seams, for long-term storage. The injected CO2 is stored in porous rock formations trapped beneath impermeable cap rocks, where it is securely contained and prevented from migrating back to the surface. Over time, the CO2 may mineralize or dissolve into underground water formations, further enhancing storage security.
CCS has the potential to significantly reduce CO2 emissions from industrial processes and power generation, helping to mitigate climate change and meet emissions reduction targets. It can also enable the continued use of fossil fuels while transitioning to a low-carbon energy future. However, CCS faces several challenges, including high costs, technological barriers, regulatory and policy uncertainties, and public acceptance issues.
Ongoing research, development, and demonstration efforts are focused on addressing these challenges and advancing CCS technologies to improve efficiency, reduce costs, and increase deployment at scale. With continued investment and support, CCS has the potential to play a crucial role in decarbonizing energy-intensive industries and achieving global climate goals.
Energy Efficiency:
Energy efficiency refers to the ratio of useful energy output to energy input in a system, process, or device. It measures the effectiveness of utilizing energy resources to achieve desired outcomes while minimizing waste and losses. Improving energy efficiency is a fundamental strategy for reducing energy consumption, lowering costs, and mitigating environmental impacts associated with energy production and consumption.
There are various ways to improve energy efficiency across different sectors, including buildings, transportation, industry, and agriculture. Some common strategies and technologies for enhancing energy efficiency include:
- Building Insulation: Proper insulation of buildings reduces heat transfer between the interior and exterior, minimizing the need for heating and cooling energy. This can be achieved through insulation materials, double-glazed windows, and sealing air leaks.
- Energy-Efficient Appliances: Energy-efficient appliances, such as refrigerators, washing machines, and air conditioners, consume less energy while providing the same level of performance as conventional models. These appliances are designed to meet stringent energy efficiency standards and often feature advanced technologies such as inverter motors and LED lighting.
- LED Lighting: Light-emitting diode (LED) lighting is more energy-efficient and longer-lasting than traditional incandescent and fluorescent lighting. LEDs consume less electricity to produce the same amount of light, resulting in significant energy savings and reduced maintenance costs.
- Vehicle Efficiency: Improving the fuel efficiency of vehicles reduces fuel consumption and greenhouse gas emissions. This can be achieved through technological advancements such as hybrid and electric vehicles, aerodynamic designs, and lightweight materials.
- Industrial Processes Optimization: Industrial facilities can optimize their processes to minimize energy waste and improve overall efficiency. This may include upgrading equipment, implementing energy management systems, and optimizing production schedules.
- Cogeneration (Combined Heat and Power): Cogeneration systems simultaneously generate electricity and useful heat from a single fuel source, maximizing energy efficiency compared to separate generation of electricity and heat. These systems are commonly used in industrial facilities, hospitals, and district heating systems.
- Smart Grids and Energy Management Systems: Smart grids and energy management systems enable real-time monitoring and control of energy usage, allowing for better optimization of energy distribution and consumption. This helps utilities and consumers make informed decisions to improve efficiency and reliability.
- Renewable Energy Integration: Integrating renewable energy sources, such as solar photovoltaics and wind turbines, into the energy system can improve overall efficiency and reduce reliance on fossil fuels. Smart grid technologies facilitate the integration of variable renewable energy sources by balancing supply and demand in real time.
- Behavioral Changes and Education: Educating consumers and promoting energy-saving behaviors can lead to significant energy savings. Simple actions such as turning off lights when not in use, adjusting thermostats, and using energy-efficient appliances can collectively make a difference in energy consumption.
By implementing energy efficiency measures and technologies, individuals, businesses, and governments can reduce energy waste, lower energy bills, and contribute to a more sustainable and resilient energy future. Moreover, improving energy efficiency plays a crucial role in achieving climate goals by reducing greenhouse gas emissions and mitigating the impacts of climate change.
Gasification:
Gasification is a thermochemical conversion process that converts carbonaceous materials, such as biomass, coal, or waste, into synthesis gas (syngas), a mixture primarily consisting of hydrogen (H2) and carbon monoxide (CO), along with other gases such as carbon dioxide (CO2), methane (CH4), and trace amounts of tar and particulates. The process occurs in a gasifier, a high-temperature reactor operating under controlled oxygen-starved conditions.
The gasification process typically involves the following steps:
- Feedstock Preparation: The carbonaceous feedstock, which can vary from coal and biomass to municipal solid waste and even plastics, is prepared for gasification. This may involve shredding, drying, and sizing the feedstock to optimize its handling and conversion in the gasifier.
- Gasification Reaction: The prepared feedstock is fed into the gasifier, where it undergoes a series of chemical reactions in a high-temperature environment (typically between 700°C and 1,500°C) with a controlled amount of oxygen (or air) and steam. The primary reactions involved in gasification are pyrolysis, oxidation, and reduction:
- Pyrolysis: The feedstock is heated in the absence of oxygen, leading to the breakdown of complex organic molecules into smaller hydrocarbons, char, and volatiles.
- Oxidation: The volatiles and char react with a limited supply of oxygen to produce CO2 and additional heat.
- Reduction: The remaining CO2 reacts with carbon (char) and water vapor (steam) to produce CO and H2, known as syngas.
- Syngas Cleanup: The raw syngas produced from the gasification process contains impurities such as tars, particulates, sulfur compounds, and trace contaminants. These impurities need to be removed to meet quality specifications for downstream applications. Syngas cleanup processes may include filtration, scrubbing, catalytic conversion, and tar cracking.
- Syngas Utilization: The cleaned syngas can be utilized in various applications, depending on its composition and quality. Common applications of syngas include:
- Power Generation: Syngas can be combusted in gas turbines, internal combustion engines, or combined-cycle power plants to generate electricity.
- Chemical Synthesis: Syngas serves as a versatile feedstock for producing a wide range of chemicals and fuels through processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia production.
- Hydrogen Production: Syngas can be further processed through water-gas shift reactions or membrane separation to produce hydrogen for fuel cells, refineries, and chemical processes.
- Biofuel Production: Syngas derived from biomass gasification can be converted into liquid biofuels such as ethanol, biodiesel, and synthetic gasoline through thermochemical or biochemical conversion pathways.
Gasification offers several advantages over conventional combustion technologies, including higher efficiency, lower emissions, and greater fuel flexibility. It enables the utilization of a wide range of feedstocks, including low-quality coal, biomass residues, and waste materials, while producing a clean and versatile syngas for energy and chemical applications. However, gasification also poses technical challenges related to process integration, syngas cleanup, and cost-effectiveness, which require ongoing research and development efforts to overcome.
Biomass Gasification:
Biomass gasification is a thermochemical process that converts biomass, such as wood, agricultural residues, and organic waste, into a combustible gas known as syngas (synthesis gas). Unlike combustion, which directly burns biomass to produce heat, gasification occurs in a controlled environment with limited oxygen supply, resulting in the partial oxidation of biomass to generate syngas. This process typically involves several stages:
- Drying: Biomass feedstock usually contains moisture, which must be removed to improve the efficiency of the gasification process. Drying the biomass reduces energy losses associated with evaporating water during gasification.
- Pyrolysis: As the biomass is heated in the absence of oxygen, it undergoes pyrolysis, a thermochemical decomposition process. This results in the release of volatile compounds, such as methane, ethylene, and tars, along with the formation of char. The volatiles and char are then subjected to further reactions to produce syngas.
- Gasification: The pyrolysis products, along with steam and a controlled amount of oxygen or air, are introduced into a gasifier reactor. Within the gasifier, high temperatures (typically between 700°C and 1,500°C) and controlled oxygen levels promote the conversion of volatiles and char into syngas through a series of chemical reactions, including:
- Reduction: Carbon dioxide (CO2) and water vapor (H2O) react with carbon (C) in the char to produce carbon monoxide (CO) and hydrogen (H2) gases through endothermic reactions.
- Water-Gas Shift: Carbon monoxide reacts with water vapor to form additional hydrogen and carbon dioxide through the water-gas shift reaction, enhancing the hydrogen content of the syngas.
- Methanation: Trace amounts of carbon monoxide and carbon dioxide may further react with hydrogen to produce methane (CH4) through methanation reactions.
- Syngas Cleanup: The raw syngas produced from biomass gasification contains impurities such as tars, particulates, ammonia, and sulfur compounds, which must be removed to meet quality specifications for downstream applications. Syngas cleanup processes include filtration, scrubbing, catalytic conversion, and tar cracking.
- Syngas Utilization: The cleaned syngas can be utilized for various energy and chemical applications, including:
- Power Generation: Syngas can be combusted in gas turbines, internal combustion engines, or combined-cycle power plants to generate electricity.
- Heat Production: Syngas can be utilized for heating applications in industrial processes, district heating systems, and residential heating.
- Biofuels Production: Syngas can serve as a feedstock for producing liquid biofuels such as ethanol, biodiesel, and synthetic gasoline through thermochemical or biochemical conversion pathways.
- Chemical Synthesis: Syngas serves as a versatile platform for producing a wide range of chemicals and fuels through processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia production.
Biomass gasification offers several advantages, including the utilization of renewable and sustainable feedstocks, reduced greenhouse gas emissions compared to fossil fuels, and potential for decentralized energy production. However, challenges such as feedstock variability, syngas cleanup, and integration with existing energy infrastructure need to be addressed to realize the full potential of biomass gasification technologies. Ongoing research and development efforts aim to improve process efficiency, reduce costs, and enhance the environmental sustainability of biomass gasification systems.
Gasification Plant:
A gasification plant is an industrial facility designed to convert carbonaceous feedstocks into synthesis gas (syngas) through the gasification process. These plants play a crucial role in the production of clean energy, chemicals, and fuels from a variety of feedstocks, including coal, biomass, municipal solid waste, and industrial residues. Here’s an overview of the components and operation of a typical gasification plant:
- Feedstock Handling and Preparation: The feedstock, which can vary widely in composition and size, is received and prepared for gasification. This may involve shredding, drying, and sizing the feedstock to optimize its handling and conversion in the gasifier.
- Gasifier: The heart of the gasification plant is the gasifier, a high-temperature reactor where the feedstock undergoes thermochemical conversion to produce syngas. Gasifiers can be classified based on their operating temperature, pressure, and feedstock type. Common types of gasifiers include fixed-bed, fluidized-bed, and entrained-flow gasifiers. In the gasifier, the feedstock is subjected to controlled heat and pressure in an oxygen-starved environment, promoting the conversion of carbonaceous materials into syngas.
- Gasification Reactions: Inside the gasifier, several chemical reactions take place, including pyrolysis, oxidation, and reduction:
- Pyrolysis: The feedstock is heated in the absence of oxygen, leading to the breakdown of complex organic molecules into smaller hydrocarbons, char, and volatiles.
- Oxidation: The volatiles and char react with a limited supply of oxygen to produce carbon dioxide (CO2) and additional heat.
- Reduction: The remaining CO2 reacts with carbon (char) and water vapor (steam) to produce carbon monoxide (CO) and hydrogen (H2), known as syngas.
- Syngas Cleanup: The raw syngas produced from the gasification process contains impurities such as tars, particulates, sulfur compounds, and trace contaminants. These impurities need to be removed to meet quality specifications for downstream applications. Syngas cleanup processes may include filtration, scrubbing, catalytic conversion, and tar cracking.
- Syngas Conditioning: After cleanup, the syngas may undergo further conditioning to adjust its composition and temperature for specific applications. This may involve processes such as cooling, heat recovery, and gas composition adjustment.
- Syngas Utilization: The cleaned and conditioned syngas can be utilized in various applications, depending on its composition and quality. Common applications of syngas include:
- Power Generation: Syngas can be combusted in gas turbines, internal combustion engines, or combined-cycle power plants to generate electricity.
- Chemical Synthesis: Syngas serves as a versatile feedstock for producing a wide range of chemicals and fuels through processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia production.
- Hydrogen Production: Syngas can be further processed through water-gas shift reactions or membrane separation to produce hydrogen for fuel cells, refineries, and chemical processes.
- Biofuel Production: Syngas derived from biomass gasification can be converted into liquid biofuels such as ethanol, biodiesel, and synthetic gasoline through thermochemical or biochemical conversion pathways.
Gasification plants offer several advantages over conventional combustion technologies, including higher efficiency, lower emissions, and greater fuel flexibility. They enable the utilization of a wide range of feedstocks, including low-quality coal, biomass residues, and waste materials, while producing a clean and versatile syngas for energy and chemical applications. However, gasification also poses technical challenges related to process integration, syngas cleanup, and cost-effectiveness, which require ongoing research and development efforts to overcome.
Syngas Generator:
A syngas generator, also known as a gas generator or gas producer, is a device or system designed to produce synthesis gas (syngas) through the gasification of carbonaceous materials. Syngas generators play a crucial role in various industrial applications, providing a versatile fuel source for power generation, heating, and chemical synthesis. Here’s an in-depth look at the components and operation of a typical syngas generator:
- Feedstock Handling: Syngas generators can utilize a wide range of feedstocks, including coal, biomass, municipal solid waste, and industrial residues. The feedstock is typically processed and prepared for gasification, which may involve drying, shredding, and sizing to optimize its conversion efficiency.
- Gasification Chamber: The gasification chamber is the primary reactor where the feedstock undergoes thermochemical conversion to produce syngas. Gasification can occur through various processes, including fixed-bed, fluidized-bed, or entrained-flow gasification, each offering unique advantages in terms of feedstock flexibility, efficiency, and syngas quality.
- Gasification Reactions: Inside the gasification chamber, the feedstock is subjected to controlled heat and pressure in an oxygen-starved environment, promoting several key reactions:
- Pyrolysis: The feedstock is heated in the absence of oxygen, leading to the release of volatile compounds and the formation of char.
- Oxidation: The volatiles and char react with a limited supply of oxygen to produce carbon monoxide (CO) and hydrogen (H2) gases through endothermic reactions.
- Reduction: Carbon monoxide and hydrogen, along with any residual carbon dioxide (CO2) and water vapor (H2O), react with char to produce additional CO and H2 through exothermic reactions.
- Heat Management: Gasification is a highly exothermic process that generates significant amounts of heat. Efficient heat management is essential to maintain optimal gasification temperatures and prevent thermal runaway. Heat may be recovered and utilized for steam generation, process heating, or power generation to enhance overall system efficiency.
- Syngas Cleanup: The raw syngas produced from gasification contains impurities such as tars, particulates, sulfur compounds, and trace contaminants. Syngas cleanup processes, including filtration, scrubbing, catalytic conversion, and tar cracking, are employed to remove these impurities and meet quality specifications for downstream applications.
- Syngas Conditioning: The cleaned syngas may undergo further conditioning to adjust its composition and temperature for specific end uses. This may involve processes such as cooling, heat recovery, gas composition adjustment, and moisture removal to ensure compatibility with downstream equipment and processes.
- Syngas Utilization: The conditioned syngas can be utilized in a variety of applications, including:
- Power Generation: Syngas can be combusted in gas turbines, internal combustion engines, or combined-cycle power plants to generate electricity.
- Heat Production: Syngas can be utilized for industrial heating applications, district heating systems, or residential heating.
- Chemical Synthesis: Syngas serves as a versatile feedstock for producing a wide range of chemicals and fuels through processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia production.
Syngas generators offer several advantages, including fuel flexibility, high efficiency, and reduced environmental impact compared to conventional combustion technologies. However, challenges such as feedstock variability, syngas cleanup, and integration with existing infrastructure need to be addressed to maximize the performance and reliability of syngas generation systems. Ongoing research and development efforts aim to improve process efficiency, reduce costs, and enhance the sustainability of syngas generation technologies.
Pyrolysis Plant:
A pyrolysis plant is an industrial facility designed to thermally decompose organic materials in the absence of oxygen to produce biochar, bio-oil, and syngas. Pyrolysis is a thermochemical process that breaks down biomass, plastic waste, tires, and other carbonaceous materials into valuable products through high-temperature heating. Here’s an in-depth exploration of the components and operation of a typical pyrolysis plant:
- Feedstock Preparation: The feedstock, which can include biomass residues, agricultural waste, plastic waste, or tires, undergoes preprocessing to remove contaminants and prepare it for pyrolysis. This may involve shredding, drying, and sizing to ensure uniformity and optimal performance during the pyrolysis process.
- Pyrolysis Reactor: The pyrolysis reactor is the core component of the plant where the feedstock is subjected to high temperatures in the absence of oxygen. The reactor can take various forms, including rotary kilns, fixed-bed reactors, fluidized-bed reactors, and auger reactors, depending on the feedstock and desired products.
- Pyrolysis Process: Inside the reactor, the feedstock undergoes thermal decomposition through several key reactions:
- Drying: Moisture present in the feedstock is evaporated at lower temperatures.
- Pyrolysis: At elevated temperatures (typically between 300°C and 800°C), the organic materials break down into volatile gases, liquids (bio-oil), and solid char (biochar) in the absence of oxygen.
- Gasification: Some of the volatile gases produced during pyrolysis undergo further thermal decomposition to form syngas, which consists primarily of carbon monoxide (CO), hydrogen (H2), methane (CH4), and other light hydrocarbons.
- Product Recovery: The pyrolysis products are separated and recovered using various methods:
- Biochar: The solid char produced during pyrolysis, known as biochar, is recovered and cooled before being stored or further processed for use as a soil amendment, carbon sequestration agent, or energy source.
- Bio-oil: The liquid fraction obtained from pyrolysis, called bio-oil or pyrolysis oil, is typically separated from the gas and solid phases using condensation or solvent extraction techniques. Bio-oil can be refined and upgraded into fuels, chemicals, or value-added products.
- Syngas: The volatile gases generated during pyrolysis and gasification are collected and cleaned to remove impurities such as tars, particulates, and sulfur compounds. The syngas can be used directly as a fuel for heat and power generation or further processed into hydrogen, methane, or synthetic fuels.
- Emissions Control: Pyrolysis plants incorporate emissions control systems to minimize air pollutants, including particulate matter, volatile organic compounds (VOCs), and greenhouse gases. Techniques such as cyclones, scrubbers, and catalytic converters are employed to capture and treat emissions before discharge into the atmosphere.
- Process Integration: Pyrolysis plants can be integrated with other facilities or processes to maximize efficiency and resource utilization. For example, waste heat from pyrolysis reactors can be recovered and used for drying feedstock, preheating combustion air, or generating steam for onsite operations.
Pyrolysis plants offer a sustainable solution for converting organic waste into valuable products, including biochar, bio-oil, and syngas, while reducing reliance on fossil fuels and mitigating environmental impacts. However, challenges such as feedstock variability, product quality control, and economic viability need to be addressed to realize the full potential of pyrolysis technology in the transition to a circular economy. Ongoing research and development efforts focus on optimizing process parameters, improving product yields and quality, and scaling up pyrolysis technologies for commercial deployment.
Pyrolysis Gas:
Pyrolysis gas, also known as pyrolysis syngas or pyrogas, is a mixture of gases produced during the thermal decomposition of organic materials in the absence of oxygen. This process, known as pyrolysis, typically occurs at temperatures between 300°C and 800°C, resulting in the breakdown of biomass, plastic waste, tires, or other carbonaceous materials into volatile gases.
The composition of pyrolysis gas varies depending on factors such as feedstock type, pyrolysis conditions, and reactor design. However, pyrolysis gas generally consists of a mixture of combustible gases, including carbon monoxide (CO), hydrogen (H2), methane (CH4), and light hydrocarbons such as ethylene (C2H4) and propylene (C3H6). Additionally, pyrolysis gas may contain small amounts of carbon dioxide (CO2), water vapor (H2O), nitrogen (N2), and trace impurities such as sulfur compounds and particulates.
The production of pyrolysis gas involves several key steps:
- Pyrolysis: During pyrolysis, organic materials are heated in the absence of oxygen, causing them to thermally decompose into volatile gases, liquids (bio-oil), and solid char (biochar). The volatile gases released during pyrolysis constitute the primary components of pyrolysis gas.
- Gasification: In addition to the gases generated directly from pyrolysis, some of the volatile components undergo further thermal decomposition through gasification reactions. This process increases the yield of hydrogen and carbon monoxide in the gas stream, enhancing the energy content and utility of the pyrolysis gas.
- Product Recovery: Pyrolysis gas is typically collected from the pyrolysis reactor and subjected to further processing to remove impurities and separate the individual gas components. Techniques such as cooling, condensation, and scrubbing are used to recover and purify the pyrolysis gas before it is used as a fuel or further processed into valuable products.
Pyrolysis gas has numerous applications across various industries:
- Energy Generation: Pyrolysis gas can be used directly as a fuel for heat and power generation in combustion engines, gas turbines, or boilers. The high energy content and clean-burning characteristics of pyrolysis gas make it a viable alternative to fossil fuels for decentralized energy production.
- Chemical Synthesis: The hydrogen and carbon monoxide present in pyrolysis gas can be utilized as feedstocks for chemical synthesis processes, such as Fischer-Tropsch synthesis, methanol production, or ammonia synthesis. These processes enable the conversion of renewable resources into valuable chemicals and fuels, contributing to the development of a sustainable bioeconomy.
- Biorefining: Pyrolysis gas can be integrated into biorefining processes to produce a wide range of value-added products, including biofuels, biochemicals, and bioplastics. By leveraging the diverse composition of pyrolysis gas, biorefineries can optimize product yields and tailor their output to meet market demand for renewable alternatives to petroleum-derived products.
Overall, pyrolysis gas represents a versatile and renewable resource with the potential to contribute to the transition towards a more sustainable and circular economy. Continued research and development efforts aim to optimize pyrolysis processes, enhance gas quality, and expand the range of applications for pyrolysis gas in the pursuit of a greener and more resilient energy future.
Pyrolysis Oil:
Pyrolysis oil, also known as bio-oil or biocrude, is a dark, viscous liquid produced through the thermal decomposition of organic materials in the absence of oxygen. This process, called pyrolysis, breaks down biomass such as wood, agricultural residues, or organic waste into a liquid product rich in organic compounds.
The production of pyrolysis oil involves heating biomass to temperatures typically ranging from 300°C to 600°C in a low-oxygen environment. Under these conditions, the complex polymers present in biomass undergo thermal degradation, yielding a mixture of liquid, gas, and solid products. Pyrolysis oil is separated from the pyrolysis gas and solid char through processes such as condensation and filtration.
The composition of pyrolysis oil varies depending on factors such as the feedstock used, pyrolysis conditions, and processing methods. However, typical components of pyrolysis oil include water, oxygenated compounds (e.g., acids, aldehydes, ketones, phenols), hydrocarbons (e.g., aromatics, olefins, paraffins), and small amounts of nitrogen and sulfur compounds.
Pyrolysis oil has several potential applications:
- Bioenergy: Pyrolysis oil can be used as a renewable fuel for heat and power generation. It can be directly combusted in boilers or furnaces to produce steam or hot water for industrial processes or district heating systems. Alternatively, it can be upgraded through processes such as hydrodeoxygenation to produce biofuels with properties similar to petroleum-derived fuels.
- Chemical Feedstock: The organic compounds present in pyrolysis oil can serve as precursors for the production of value-added chemicals and materials. Through processes such as catalytic cracking, hydrogenation, or esterification, pyrolysis oil can be converted into fuels, lubricants, solvents, resins, or specialty chemicals used in various industries.
- Biochar Production: The solid char residue generated during pyrolysis, known as biochar, can be used as a soil amendment to improve soil fertility, water retention, and carbon sequestration. Biochar can be produced alongside pyrolysis oil and gas, providing an integrated approach to biomass utilization and soil management.
Despite its potential, pyrolysis oil faces challenges related to its high oxygen content, low energy density, and instability over time. Research efforts are focused on developing advanced pyrolysis technologies, optimizing process conditions, and refining upgrading techniques to enhance the quality and utility of pyrolysis oil for various applications.
Overall, pyrolysis oil represents a promising avenue for converting biomass into valuable products, contributing to the development of a sustainable bioeconomy and reducing reliance on fossil fuels. Continued innovation and investment in pyrolysis technology hold the key to unlocking the full potential of pyrolysis oil as a renewable and versatile resource.
Gasification Plant:
A gasification plant is a facility designed to convert carbonaceous feedstocks such as coal, biomass, or waste into a synthesis gas (syngas) through the process of gasification. Gasification is a thermochemical conversion process that occurs in a controlled environment, typically at elevated temperatures and pressures, in the presence of a controlled amount of oxygen or steam.
The key components of a gasification plant include:
- Feedstock Preparation: Carbonaceous feedstocks, such as coal, wood chips, agricultural residues, or municipal solid waste, are prepared and conditioned before entering the gasification reactor. This may involve shredding, drying, and size reduction to optimize feedstock characteristics for efficient gasification.
- Gasification Reactor: The gasification reactor is the core component of the plant where the conversion of feedstock into syngas takes place. Various reactor designs are employed, including fixed-bed, fluidized-bed, and entrained-flow reactors, each offering different advantages in terms of feedstock flexibility, residence time, and gasification efficiency. The reactor operates at temperatures typically ranging from 700°C to 1500°C, depending on the feedstock and desired syngas composition.
- Gasification Agent: Gasification can be carried out using different gasification agents, such as air, oxygen, or steam, depending on the desired product and process conditions. Air-blown gasification is commonly used for low-grade feedstocks like coal, while oxygen-blown or steam-blown gasification is preferred for biomass or waste materials to minimize the production of undesirable by-products such as nitrogen oxides or tars.
- Syngas Cleanup: The raw syngas produced in the gasification reactor contains impurities such as particulates, tar, sulfur compounds, and trace contaminants. Syngas cleanup processes, including filtration, scrubbing, cooling, and catalytic conversion, are employed to remove impurities and improve the quality of the syngas before downstream utilization or storage.
- Syngas Utilization: The cleaned syngas can be utilized for a variety of applications, including heat and power generation, production of chemicals, fuels, or fertilizers, or as a feedstock for downstream processes such as Fischer-Tropsch synthesis or methanol production. Gasification offers flexibility in syngas composition, allowing for the production of a wide range of products tailored to specific market demands.
Gasification plants offer several advantages over traditional combustion-based power generation, including higher efficiency, lower emissions, and greater fuel flexibility. Additionally, gasification enables the utilization of a wide range of feedstocks, including low-grade coals, biomass residues, or waste materials, contributing to resource diversification and waste valorization.
However, gasification technology also poses challenges related to process complexity, feedstock handling, and syngas cleanup. Research and development efforts focus on improving gasification efficiency, enhancing feedstock flexibility, and reducing capital and operating costs to make gasification more economically viable and environmentally sustainable.
Overall, gasification plants play a crucial role in the transition towards a more sustainable and resilient energy future by enabling the efficient conversion of diverse carbonaceous resources into clean and versatile syngas for various industrial, commercial, and residential applications.
Syngas Generator:
A syngas generator is a device or system designed to produce synthesis gas (syngas) from various carbonaceous feedstocks through the process of gasification. Syngas generators are integral components of gasification plants and play a crucial role in converting solid or liquid carbon-containing materials into a gaseous fuel or chemical precursor.
The operation of a syngas generator involves several key steps:
- Feedstock Preparation: Depending on the type of feedstock used, preparation may involve drying, shredding, and sizing to optimize its characteristics for efficient gasification. Common feedstocks include coal, biomass, municipal solid waste, or industrial residues.
- Gasification Process: The gasification process occurs within the syngas generator, typically in a high-temperature environment and in the presence of a controlled amount of oxygen, air, steam, or a combination thereof. Gasification transforms the feedstock into syngas, which is primarily composed of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and trace amounts of other gases such as methane (CH4) and nitrogen (N2).
- Gasification Reactor: Syngas generators utilize various types of gasification reactors, including fixed-bed, fluidized-bed, entrained-flow, or plasma gasifiers, each offering unique advantages in terms of feedstock flexibility, gasification efficiency, and syngas quality. The reactor design influences factors such as residence time, temperature, and gas-solid interactions during the gasification process.
- Heat Supply: Gasification requires a significant input of heat energy to drive the endothermic reactions involved in breaking down the feedstock into syngas. Heat may be supplied externally through combustion of a portion of the feedstock or through the use of auxiliary heating sources such as electrical heating elements or high-temperature gases.
- Syngas Cooling and Cleanup: The raw syngas produced in the gasification reactor is typically high-temperature and contains impurities such as tars, particulates, sulfur compounds, and trace contaminants. Syngas cooling and cleanup systems, including quenching, cyclones, filters, scrubbers, and catalytic converters, are employed to remove impurities and cool the syngas to suitable temperatures for downstream processing and utilization.
- Syngas Utilization: The cleaned syngas can be utilized for a wide range of applications, including heat and power generation, production of chemicals, fuels, or fertilizers, or as a feedstock for further processing such as methanol synthesis, Fischer-Tropsch synthesis, or hydrogen production.
Syngas generators offer several advantages, including the ability to convert diverse feedstocks into a versatile fuel or chemical precursor, high energy efficiency compared to traditional combustion processes, and reduced environmental emissions. However, challenges such as feedstock variability, syngas impurities, and process complexity require ongoing research and development efforts to improve gasification technology and make syngas generation more cost-effective and environmentally sustainable.
In summary, syngas generators play a vital role in the utilization of carbonaceous resources for energy and chemical production, contributing to the transition towards a more sustainable and resilient energy future.
Thermal Conversion:
Thermal conversion refers to a set of chemical processes that transform organic materials into useful products or energy carriers through the application of heat. This term encompasses various thermochemical processes, including combustion, gasification, pyrolysis, and liquefaction, each with distinct mechanisms and product outputs.
- Combustion: Combustion is the most common form of thermal conversion, where organic materials are oxidized in the presence of oxygen to release heat energy and produce combustion products such as carbon dioxide, water vapor, and ash. This process is widely used in power plants, heating systems, and industrial furnaces to generate heat and electricity.
- Gasification: Gasification involves heating organic materials, such as coal, biomass, or waste, in a controlled environment with a limited supply of oxygen or steam. This thermochemical process produces synthesis gas (syngas), which is a mixture of hydrogen, carbon monoxide, methane, and other trace gases. Syngas can be utilized for power generation, chemical synthesis, or as a precursor for liquid fuels.
- Pyrolysis: Pyrolysis is a thermal decomposition process that occurs in the absence of oxygen, leading to the breakdown of organic materials into solid char, liquid bio-oil, and combustible gases such as hydrogen, methane, and carbon monoxide. Pyrolysis is often used to convert biomass feedstocks into biochar, bio-oil, and biogas for applications in soil amendment, biofuels production, and renewable energy generation.
- Liquefaction: Liquefaction processes convert solid or gaseous organic materials into liquid hydrocarbons through the application of heat and pressure. One example is biomass liquefaction, where biomass feedstocks are heated in the presence of solvents or catalysts to produce bio-oil or synthetic fuels that can be refined and used as transportation fuels or chemical feedstocks.
Thermal conversion technologies offer several advantages, including energy recovery from renewable resources, waste reduction, and the production of valuable products such as biofuels, chemicals, and heat. However, challenges such as high capital costs, feedstock variability, and environmental concerns related to emissions and waste management must be addressed to ensure the widespread adoption and sustainability of thermal conversion processes.
Research and development efforts focus on improving process efficiency, enhancing feedstock flexibility, and developing advanced materials and catalysts to optimize thermal conversion technologies and contribute to the transition towards a more sustainable and carbon-neutral energy future.
- Tar Removal: Tar removal is the process of eliminating tar, a complex mixture of organic compounds produced during various thermal processes such as gasification, pyrolysis, and combustion.
- Gas Cleaning: Gas cleaning involves the removal of impurities, including tar, from gases produced during thermal conversion processes to meet product specifications or environmental regulations.
- Tar Abatement: Tar abatement refers to techniques employed to reduce or eliminate the formation of tar during biomass gasification or pyrolysis, thereby improving process efficiency and product quality.
- Catalytic Tar Decomposition: Catalytic tar decomposition involves the use of catalysts to promote the chemical breakdown of tar molecules into smaller, less problematic compounds such as methane, hydrogen, and carbon monoxide.
- Tar Cracking: Tar cracking is a thermal decomposition process in which tar molecules are thermally cracked into lighter hydrocarbons and gases at elevated temperatures, typically above 800°C, in the presence of steam or catalysts.
- Tar Reforming: Tar reforming involves chemically transforming tar compounds into more desirable products through catalytic reactions, such as steam reforming, where tar is converted into hydrogen and carbon monoxide.
- Tar Filtration: Tar filtration utilizes filtration media, such as ceramic filters or porous membranes, to physically capture tar particles from gas streams, preventing their entry into downstream equipment or the environment.
- Tar Condensation: Tar condensation involves cooling gas streams containing tar compounds to temperatures below their dew point, causing tars to condense into liquid form for subsequent removal through separation processes.
- Tar Scrubbing: Tar scrubbing employs liquid solvents or scrubbing agents to absorb tar compounds from gas streams through chemical or physical interactions, facilitating their removal from the gas phase.
- Tar Hydrocracking: Tar hydrocracking involves subjecting tar-rich streams to high-pressure, high-temperature conditions in the presence of hydrogen and catalysts to break down tar molecules into lighter hydrocarbons suitable for further processing.
- Tar Pyrolysis: Tar pyrolysis is a thermal decomposition process where tar is subjected to high temperatures in the absence of oxygen, leading to its decomposition into simpler, more valuable products such as bio-oil or syngas.
- Tar Reforming: Tar reforming employs catalytic processes to convert tar compounds into synthesis gas (syngas), which consists primarily of hydrogen and carbon monoxide, suitable for use in various chemical synthesis or energy generation applications.
- Tar Fractionation: Tar fractionation separates tar compounds based on their molecular weight or chemical properties, allowing for the isolation of specific fractions for further treatment or utilization.
- Tar Adsorption: Tar adsorption involves the use of adsorbent materials, such as activated carbon or zeolites, to selectively adsorb tar compounds from gas streams, facilitating their removal and recovery.
- Tar Destruction: Tar destruction technologies, such as thermal oxidation or plasma gasification, involve subjecting tar-containing streams to high temperatures and oxidative environments to completely oxidize tar compounds into harmless gases such as carbon dioxide and water vapor.
- Tar Hydrogenation: Tar hydrogenation processes involve reacting tar compounds with hydrogen under high-pressure conditions in the presence of catalysts to convert tars into liquid hydrocarbons or other value-added products.
- Tar Gasification: Tar gasification converts tar compounds into syngas through partial oxidation or steam reforming processes, enabling their utilization as a fuel or chemical feedstock in various industrial applications.
- Tar Stabilization: Tar stabilization techniques aim to chemically or physically modify tar compounds to reduce their reactivity or volatility, thereby minimizing their detrimental effects on downstream equipment or processes.
- Tar Removal Efficiency: Tar removal efficiency refers to the effectiveness of tar removal technologies in achieving the desired reduction of tar content in gas streams, often expressed as a percentage of tar removed relative to the initial tar concentration.
- Tar Management: Tar management encompasses the selection and integration of appropriate tar removal technologies into thermal conversion processes to ensure efficient operation, product quality, and compliance with environmental regulations.
Tar Removal:
Tar removal is a critical process in the realm of thermal conversion technologies, particularly in biomass gasification, pyrolysis, and combustion systems. Tar, a complex mixture of organic compounds, poses significant challenges in these processes due to its detrimental effects on equipment, product quality, and environmental emissions.
During biomass gasification, for example, tar can condense on equipment surfaces, leading to fouling and corrosion, thereby reducing system efficiency and reliability. Moreover, tar can also cause blockages in downstream pipes and filters, resulting in operational disruptions and increased maintenance costs.
In biomass pyrolysis, tar can lower the quality of bio-oil produced, affecting its usability as a biofuel or chemical feedstock. Additionally, tar can contribute to particulate matter emissions during combustion, leading to air pollution and environmental concerns.
To address these challenges, various tar removal technologies have been developed, ranging from physical separation methods to chemical and catalytic processes. Physical methods such as tar filtration and condensation involve cooling the gas stream to condense tar compounds, which can then be separated from the gas phase. Additionally, tar scrubbing utilizes liquid solvents or scrubbing agents to absorb tar molecules from the gas stream, facilitating their removal.
Chemical and catalytic tar removal processes involve the decomposition, reforming, or conversion of tar compounds into less problematic or more valuable products. Catalytic tar cracking, for instance, employs catalysts to break down tar molecules into smaller, lighter hydrocarbons or gases, which can then be further processed or utilized. Similarly, tar reforming reactions can transform tar compounds into synthesis gas (syngas), a versatile energy carrier used in power generation, chemical synthesis, and fuel production.
The choice of tar removal technology depends on various factors, including the composition of the feedstock, process conditions, desired product specifications, and economic considerations. Additionally, integration with other process units and system optimization play crucial roles in achieving efficient tar removal and overall process performance.
In summary, tar removal is a critical aspect of thermal conversion processes, essential for ensuring equipment integrity, product quality, and environmental compliance. Ongoing research and development efforts continue to advance tar removal technologies, aiming to enhance efficiency, reduce costs, and promote the widespread adoption of renewable energy technologies.
Gas Cleaning:
Gas cleaning is an indispensable process employed in various industrial applications to remove impurities and contaminants from gas streams, ensuring compliance with environmental regulations, protecting downstream equipment, and improving product quality. Gas cleaning is particularly crucial in thermal conversion processes such as biomass gasification, pyrolysis, and combustion, where the presence of impurities, including particulate matter, tar, sulfur compounds, and volatile organic compounds (VOCs), can have adverse effects on process efficiency and environmental emissions.
In the context of biomass gasification, for example, gas cleaning plays a vital role in removing tar, ash, and other contaminants from the syngas produced, making it suitable for downstream applications such as power generation, chemical synthesis, or biofuel production. Similarly, in biomass pyrolysis, gas cleaning is essential for purifying the bio-oil and syngas streams, enhancing their usability as renewable fuels or feedstocks.
Gas cleaning technologies encompass a wide range of methods, including mechanical filtration, electrostatic precipitation, cyclone separation, wet scrubbing, adsorption, catalytic conversion, and thermal oxidation. Mechanical filtration utilizes filters or porous membranes to physically capture particulate matter and other solid contaminants from the gas stream. Electrostatic precipitation involves the use of electrostatic forces to charge and collect particulates on charged plates or electrodes, facilitating their removal from the gas phase.
Wet scrubbing employs liquid scrubbing agents, such as water or chemical solutions, to chemically or physically absorb contaminants from the gas stream. Adsorption techniques utilize adsorbent materials, such as activated carbon or zeolites, to selectively adsorb volatile organic compounds or other gaseous pollutants from the gas phase. Catalytic conversion processes employ catalysts to facilitate chemical reactions that convert harmful pollutants into less harmful or inert substances.
Furthermore, thermal oxidation, also known as combustion, involves subjecting the gas stream to high temperatures in the presence of excess oxygen to completely oxidize organic contaminants into carbon dioxide and water vapor, effectively eliminating them from the gas phase.
The selection of gas cleaning technologies depends on various factors, including the composition and concentration of contaminants, gas stream characteristics, process conditions, and regulatory requirements. Additionally, system integration, energy efficiency, and cost-effectiveness play crucial roles in determining the optimal gas cleaning approach for a particular application.
In conclusion, gas cleaning is an essential step in thermal conversion processes, ensuring the production of clean and environmentally friendly gases suitable for a wide range of industrial and energy applications. Ongoing advancements in gas cleaning technologies continue to drive improvements in process efficiency, environmental performance, and overall sustainability.
Syngas Conditioning:
Syngas conditioning is a crucial process in biomass gasification and other thermochemical conversion technologies aimed at producing synthesis gas (syngas) with specific composition and properties suitable for various downstream applications. Syngas, a mixture primarily composed of carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), methane (CH4), and trace contaminants such as tar, sulfur compounds, and particulate matter, requires conditioning to meet the requirements of end-use applications such as power generation, chemical synthesis, and fuel production.
The primary objectives of syngas conditioning include adjusting the syngas composition, removing impurities and contaminants, controlling temperature and pressure, and optimizing the syngas properties for downstream processes. This often involves a combination of physical, chemical, and catalytic processes tailored to the specific requirements of the application.
One of the key steps in syngas conditioning is tar removal, as tar compounds can be detrimental to downstream equipment and catalysts, leading to fouling and deactivation. Various tar removal methods, such as filtration, condensation, scrubbing, and catalytic cracking, are employed to reduce tar concentrations to acceptable levels.
In addition to tar removal, syngas conditioning may involve processes such as water-gas shift reaction (WGSR) to adjust the ratio of CO to H2, which is essential for applications such as methanol synthesis or ammonia production. The WGSR converts CO and water vapor into CO2 and H2, increasing the hydrogen content of the syngas.
Furthermore, syngas conditioning often includes sulfur removal to meet environmental regulations and prevent sulfur poisoning of downstream catalysts. Hydrogen sulfide (H2S) removal can be achieved through processes such as desulfurization using absorbents or catalytic hydrolysis.
Syngas conditioning may also involve adjusting the syngas temperature and pressure to meet the requirements of downstream processes. Cooling and compression units are often employed for this purpose, ensuring that the syngas is delivered at the desired temperature and pressure conditions.
Overall, syngas conditioning is a critical step in biomass gasification and other thermochemical conversion processes, ensuring that the produced syngas meets the quality standards required for various applications. Advances in syngas conditioning technologies continue to drive improvements in process efficiency, product quality, and environmental performance, enabling the widespread adoption of syngas-based renewable energy systems.
Gasification Catalysts:
Gasification catalysts play a significant role in biomass gasification processes by enhancing reaction kinetics, promoting syngas quality, and improving overall process efficiency. Gasification, a thermochemical conversion process, involves the partial oxidation of biomass feedstock to produce a synthesis gas (syngas) containing hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and traces of other gases.
The use of catalysts in gasification processes offers several advantages, including:
- Enhanced Reaction Kinetics: Catalysts facilitate the conversion of complex biomass molecules into simpler, more readily convertible intermediates, thereby accelerating gasification reactions. This results in improved process efficiency and higher syngas yields.
- Tar Reduction: Tar compounds, formed during biomass gasification, can lead to equipment fouling and catalyst deactivation. Catalysts can promote tar cracking reactions, converting tars into smaller, less problematic molecules or into additional syngas components, thereby reducing tar concentrations in the product gas.
- Syngas Composition Control: Catalysts can influence the composition of the syngas by selectively promoting certain gasification reactions over others. For example, catalysts can favor the water-gas shift reaction, which converts CO and water vapor into CO2 and H2, thereby increasing the hydrogen content of the syngas.
- Temperature Moderation: Gasification catalysts can lower the reaction temperature required for biomass conversion, allowing for more controlled and efficient operation of gasification reactors. This can mitigate issues such as thermal stress and reactor material degradation.
Gasification catalysts can be classified based on their chemical composition, structure, and mode of action. Common types of gasification catalysts include:
- Metal Catalysts: These catalysts typically consist of transition metals such as nickel, cobalt, and iron, supported on a high-surface-area substrate such as alumina, silica, or zeolites. Metal catalysts are known for their high activity and selectivity in promoting gasification reactions.
- Biomass-Derived Catalysts: Catalysts derived from biomass feedstock or biomass-derived materials offer the advantage of being renewable and environmentally friendly. These catalysts can be synthesized from biomass residues such as lignin, cellulose, or biochar, and they often exhibit unique catalytic properties suited to biomass gasification conditions.
- Mixed Metal Oxide Catalysts: Mixed metal oxide catalysts, such as ceria-zirconia or alumina-silica, possess tailored surface properties that make them effective in promoting gasification reactions. These catalysts offer high surface area, thermal stability, and resistance to poisoning, making them suitable for harsh gasification environments.
The selection of gasification catalysts depends on various factors, including the specific biomass feedstock, gasification conditions, desired syngas composition, and process economics. Ongoing research and development efforts aim to optimize catalyst formulations, improve catalyst stability, and develop novel catalyst materials to further enhance the performance and sustainability of biomass gasification processes.
Syngas Utilization:
Syngas, a versatile mixture primarily composed of carbon monoxide (CO) and hydrogen (H2), holds significant potential as a feedstock for various downstream processes aimed at producing fuels, chemicals, and other value-added products. Syngas utilization encompasses a broad spectrum of applications, ranging from traditional fuel synthesis to emerging technologies for renewable energy and sustainable chemistry.
- Fuel Synthesis: One of the primary applications of syngas is in the synthesis of liquid fuels such as methanol, synthetic natural gas (SNG), and Fischer-Tropsch (FT) diesel and gasoline. These fuels can be produced via catalytic processes that involve the conversion of syngas components into longer-chain hydrocarbons suitable for use in transportation and heating.
- Chemical Production: Syngas serves as a precursor for the production of a wide range of chemicals, including ammonia, methanol, hydrogen cyanide, and various organic acids. These chemicals are essential building blocks for industries such as fertilizers, pharmaceuticals, plastics, and textiles, among others.
- Power Generation: Syngas can be used as a fuel in gas turbines, reciprocating engines, and fuel cells for power generation. Gasification-based power plants utilize syngas to produce electricity efficiently while capturing waste heat for combined heat and power (CHP) applications, enhancing overall energy efficiency.
- Hydrogen Production: Syngas can be further processed to produce high-purity hydrogen gas, which is utilized in various industrial processes, fuel cells, and hydrogenation reactions. Hydrogen produced from syngas via water-gas shift reaction (WGSR) or membrane technologies contributes to the decarbonization of sectors such as transportation and industry.
- Biofuel Production: Syngas derived from biomass feedstock serves as a sustainable alternative to fossil fuels for biofuel production. Biomass-to-liquid (BTL) processes convert syngas into biofuels such as bioethanol, biodiesel, and renewable aviation fuels, offering lower greenhouse gas emissions and reduced dependence on finite fossil resources.
- Chemical Synthesis: Syngas can be utilized for the synthesis of specialty chemicals, solvents, and intermediates used in pharmaceutical, agrochemical, and specialty chemical industries. Tailored catalytic processes enable the conversion of syngas into specific chemical products with high selectivity and yield.
- Renewable Energy Storage: Syngas can serve as a form of renewable energy storage by converting surplus electricity from intermittent renewable sources such as wind and solar power into storable chemical energy in the form of hydrogen or synthetic fuels. This stored energy can be later converted back into electricity or used as a fuel for transportation and heating.
Syngas utilization technologies continue to evolve, driven by advancements in catalysis, process engineering, and sustainability considerations. Integration of syngas utilization pathways with renewable energy sources and carbon capture and utilization (CCU) strategies offers promising opportunities for reducing greenhouse gas emissions, mitigating climate change, and transitioning towards a more sustainable energy and chemical industry.
Syngas Cleaning and Conditioning:
Syngas produced from biomass gasification processes often contains impurities such as tars, particulates, sulfur compounds, ammonia, and trace contaminants that can degrade downstream equipment, catalysts, and product quality. Syngas cleaning and conditioning technologies are employed to remove these impurities and improve syngas quality for subsequent utilization in various applications.
- Tar Removal: Tar compounds, formed during the pyrolysis and gasification of biomass feedstock, are complex organic compounds that can condense and solidify at lower temperatures, leading to fouling of downstream equipment and catalyst deactivation. Tar removal technologies include tar cracking, tar filtration, and tar reforming processes, which aim to decompose tars into simpler, more volatile compounds or remove them from the syngas stream through filtration or absorption.
- Particulate Removal: Particulate matter, consisting of ash, char, and unconverted biomass residues, can cause erosion and abrasion in gasification reactors and downstream equipment. Particulate removal technologies such as cyclone separators, ceramic filters, and electrostatic precipitators are employed to capture and remove solid particles from the syngas stream, thereby protecting downstream components and improving syngas quality.
- Sulfur Removal: Sulfur compounds present in biomass feedstock or formed during gasification processes can poison catalysts and contribute to environmental pollution. Syngas cleaning technologies such as desulfurization reactors, adsorption beds, and chemical scrubbers are utilized to remove sulfur-containing compounds such as hydrogen sulfide (H2S) and carbonyl sulfide (COS) from the syngas stream, ensuring compliance with environmental regulations and enhancing process efficiency.
- Ammonia Removal: Ammonia (NH3) is often present in syngas as a byproduct of biomass decomposition and nitrogen-containing compounds in the feedstock. Ammonia can corrode equipment and catalytic surfaces and affect downstream processes. Ammonia removal technologies such as selective catalytic reduction (SCR) and ammonia scrubbing are employed to convert or absorb ammonia from the syngas stream, improving product purity and reducing environmental impact.
- Particulate Filtration: Syngas conditioning systems often include particulate filtration units such as cyclone separators, fabric filters, or ceramic filters to remove solid particles, ash, and char from the syngas stream. These filtration systems operate based on principles of inertia, gravity, or electrostatic attraction to capture and separate particulate matter, ensuring clean syngas for downstream applications.
- Hydrogen Sulfide Removal: Hydrogen sulfide (H2S) is a common impurity in syngas produced from biomass gasification processes, originating from sulfur-containing compounds in the biomass feedstock. H2S removal technologies include chemical scrubbing with amine-based solvents, adsorption onto solid sorbents, and catalytic oxidation to convert H2S into elemental sulfur or sulfur dioxide (SO2), ensuring compliance with environmental regulations and preventing catalyst poisoning.
- Gas Cooling and Condensation: Syngas is typically hot and contains high levels of water vapor and tar precursors, which can condense and form liquid aerosols at lower temperatures, leading to equipment fouling and corrosion. Gas cooling and condensation technologies involve quenching the syngas stream to lower temperatures, allowing for the removal of water vapor and condensable hydrocarbons through condensation and separation processes, thereby improving syngas quality and protecting downstream equipment.
Syngas cleaning and conditioning technologies are critical for ensuring the reliable operation, performance, and environmental sustainability of biomass gasification processes. Integration of advanced cleaning and conditioning systems enables the production of clean syngas streams with high purity and quality, suitable for a wide range of applications in renewable energy, chemicals, and fuels production.
Sulfur Removal in Syngas:
Sulfur removal is a crucial step in the cleaning and conditioning of syngas generated from biomass gasification processes. Sulfur compounds such as hydrogen sulfide (H2S) and carbonyl sulfide (COS) are commonly found in syngas as byproducts of sulfur-containing compounds present in biomass feedstock or formed during gasification reactions. These sulfur compounds are highly corrosive and can poison catalysts, thereby negatively impacting downstream processes and product quality. Therefore, effective sulfur removal is essential to ensure the integrity of equipment, compliance with environmental regulations, and the quality of syngas for various applications.
- Hydrogen Sulfide (H2S) Removal: Hydrogen sulfide is a colorless, toxic gas with a characteristic rotten egg odor. It is often present in syngas streams at varying concentrations depending on the sulfur content of the biomass feedstock and gasification conditions. H2S removal is typically achieved through chemical scrubbing using aqueous solutions of alkaline compounds such as sodium hydroxide (NaOH) or amine-based solvents such as monoethanolamine (MEA) or diethanolamine (DEA). In the scrubbing process, the syngas is contacted with the scrubbing solution, where H2S reacts with the alkaline compounds to form stable sulfide salts, which are then removed from the syngas stream. The scrubbed syngas is then dried and further processed to remove any remaining impurities before utilization.
- Catalytic Hydrogenation: Catalytic hydrogenation is another method employed for H2S removal from syngas. In this process, syngas is passed over a catalyst bed typically containing transition metal sulfides such as nickel or cobalt supported on alumina or other suitable carriers. H2S reacts with hydrogen (H2) over the catalyst surface to form water (H2O) and elemental sulfur (S), which can be subsequently removed from the syngas stream. The catalytic hydrogenation process operates at elevated temperatures and pressures and requires the presence of a hydrogen source, making it suitable for integrated gasification combined cycle (IGCC) plants where hydrogen is readily available.
- Adsorption: Adsorption technologies involve the use of solid sorbents to capture and remove sulfur compounds from the syngas stream. Activated carbon, zeolites, metal oxides, and other porous materials are commonly used as adsorbents due to their high surface area and affinity for sulfur species. Syngas is passed through a fixed bed or fluidized bed of adsorbent material, where sulfur compounds are selectively adsorbed onto the surface of the sorbent. The adsorbent can be regenerated periodically by desorption using heat or chemical agents, allowing for continuous operation of the adsorption process.
- Biological Desulfurization: Biological desulfurization, also known as biodesulfurization, is an emerging technology that utilizes sulfur-oxidizing bacteria or fungi to oxidize and convert sulfur compounds in syngas into water-soluble sulfate ions. The process operates under mild conditions and does not require the use of hazardous chemicals or high temperatures. Biological desulfurization can be integrated into gasification systems as a biological polishing step to further reduce sulfur content in syngas, offering a sustainable and environmentally friendly approach to sulfur removal.
Sulfur removal technologies play a critical role in ensuring the quality and purity of syngas produced from biomass gasification processes. By effectively removing sulfur compounds, these technologies enable the utilization of syngas in various applications, including power generation, chemical synthesis, and fuel production, while minimizing environmental impact and ensuring compliance with stringent emissions regulations.
Biomass Gasification Process:
Biomass gasification is a thermochemical conversion process that converts biomass feedstock into a gaseous fuel called syngas (synthesis gas), which primarily consists of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and traces of other gases such as methane (CH4) and nitrogen (N2). This process involves the partial oxidation of biomass in a controlled environment, typically in the presence of a gasifying agent such as air, oxygen, or steam, at elevated temperatures ranging from 600°C to 1,200°C.
- Feedstock Preparation: Biomass feedstock, which can include a variety of organic materials such as wood chips, agricultural residues, energy crops, and municipal solid waste, undergoes size reduction and drying processes to achieve uniform particle size distribution and moisture content suitable for gasification. Proper feedstock preparation ensures efficient gasification and prevents issues such as bridging and channeling in the gasifier.
- Gasification Reactor: The gasification reactor is the core component of the biomass gasification system where biomass feedstock undergoes thermochemical conversion to produce syngas. Gasifiers can be classified based on the type of gasification process, including fixed-bed, fluidized-bed, entrained-flow, and downdraft gasifiers. Each type of gasifier operates under specific temperature, pressure, and residence time conditions to optimize gasification efficiency and syngas composition.
- Pyrolysis Zone: In the gasification reactor, biomass undergoes sequential thermochemical reactions starting with pyrolysis, where biomass is heated in the absence of oxygen to temperatures typically ranging from 300°C to 600°C. During pyrolysis, biomass decomposes into volatile compounds such as tar, methane, and other hydrocarbons, as well as char, a carbon-rich residue.
- Combustion Zone: Following pyrolysis, the volatiles produced enter the combustion zone of the gasifier, where they react with a controlled amount of oxygen or steam to undergo further oxidation reactions, releasing additional heat and producing carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). The exothermic combustion reactions help sustain the gasification process by providing the energy required for endothermic reactions in the reduction zone.
- Reduction Zone: In the reduction zone, carbon dioxide (CO2) and water vapor (H2O) react with the carbonaceous char produced during pyrolysis to undergo gasification reactions, primarily producing carbon monoxide (CO) and hydrogen (H2) through the water-gas shift reaction and carbon gasification reactions. The gasification reactions are facilitated by the presence of steam and a controlled amount of oxygen or air, which act as gasifying agents.
- Syngas Cleanup: The raw syngas produced from the gasification reactor contains impurities such as tars, particulates, sulfur compounds, and trace contaminants, which must be removed to meet product specifications and prevent equipment fouling and catalyst poisoning in downstream processes. Syngas cleanup technologies include tar removal, particulate filtration, sulfur removal, and trace contaminant removal through processes such as catalytic cracking, scrubbing, adsorption, and catalytic oxidation.
- Syngas Utilization: The cleaned syngas can be utilized in various applications, including power generation, heat production, chemical synthesis, and transportation fuels. Syngas can be combusted directly in gas turbines, reciprocating engines, or boilers to produce electricity and heat or further processed through catalytic conversion processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia synthesis to produce value-added fuels and chemicals.
Biomass gasification offers a versatile and sustainable pathway for converting renewable biomass resources into a clean and flexible energy carrier in the form of syngas. By optimizing gasification technologies and integrating syngas cleanup and utilization processes, biomass gasification can play a significant role in reducing greenhouse gas emissions, mitigating climate change, and enhancing energy security.
Syngas Production:
Syngas, short for synthesis gas, is a versatile fuel composed primarily of carbon monoxide (CO) and hydrogen (H2), along with other gases such as carbon dioxide (CO2), methane (CH4), and nitrogen (N2). It is produced through various processes known as gasification, which involves the partial oxidation of carbonaceous materials such as coal, biomass, or natural gas. Below is an in-depth look at syngas production:
- Gasification Process: Syngas production begins with the gasification of carbonaceous feedstock in a controlled environment, typically at high temperatures and pressures. Gasification can be carried out using various feedstocks and gasification technologies, such as fixed-bed, fluidized-bed, entrained-flow, and downdraft gasifiers. These gasifiers operate under different conditions to optimize syngas yield and composition.
- Feedstock Preparation: Prior to gasification, the carbonaceous feedstock undergoes size reduction, drying, and sometimes preprocessing steps to enhance its suitability for gasification. This ensures uniformity in particle size distribution and moisture content, facilitating efficient gasification and preventing issues such as bridging and channeling within the gasifier.
- Thermochemical Reactions: Inside the gasifier, the feedstock undergoes thermochemical reactions in the presence of a gasifying agent, which can be air, oxygen, steam, or a combination of these. The primary reactions include pyrolysis, combustion, and gasification:
- Pyrolysis: At high temperatures in the absence of oxygen, the feedstock decomposes into volatile components such as tar, methane, and other hydrocarbons, as well as char.
- Combustion: Oxygen or air is introduced into the gasifier to combust a portion of the feedstock, generating heat and carbon dioxide.
- Gasification: The remaining carbonaceous material reacts with steam and/or carbon dioxide to produce syngas, primarily consisting of carbon monoxide and hydrogen, along with other gases.
- Syngas Composition: The composition of syngas produced depends on several factors, including the feedstock type, gasification technology, operating conditions, and the choice of gasifying agent. Typically, syngas contains varying proportions of carbon monoxide (CO) and hydrogen (H2), with lesser amounts of carbon dioxide (CO2), methane (CH4), water vapor (H2O), and nitrogen (N2).
- Syngas Cleanup: The raw syngas produced from the gasifier often contains impurities such as tars, particulates, sulfur compounds, and trace contaminants. These impurities need to be removed through syngas cleanup processes to meet product specifications and prevent equipment fouling and catalyst poisoning in downstream processes. Syngas cleanup technologies include tar removal, particulate filtration, sulfur removal, and trace contaminant removal through processes such as catalytic cracking, scrubbing, adsorption, and catalytic oxidation.
- Syngas Utilization: Once cleaned, syngas can be utilized in various applications, including power generation, heat production, chemical synthesis, and transportation fuels. Syngas can be combusted directly in gas turbines, reciprocating engines, or boilers to produce electricity and heat, or further processed through catalytic conversion processes such as Fischer-Tropsch synthesis, methanol synthesis, and ammonia synthesis to produce value-added fuels and chemicals.
In summary, syngas production involves a series of thermochemical reactions that convert carbonaceous feedstock into a versatile fuel suitable for a wide range of applications. By optimizing gasification technologies and integrating syngas cleanup and utilization processes, syngas can play a significant role in meeting energy demands while reducing environmental impact and enhancing energy security.
Gasification Catalysts:
Gasification catalysts play a crucial role in promoting and controlling the chemical reactions involved in the gasification process. They facilitate the conversion of solid carbonaceous materials into syngas by lowering the activation energy required for the reactions to occur, thus improving reaction kinetics and enhancing syngas yield and quality. Here’s an in-depth exploration of gasification catalysts:
- Catalytic Gasification: Gasification catalysts are typically employed in catalytic gasification processes, where they accelerate the conversion of feedstock into syngas at lower temperatures and pressures compared to non-catalytic gasification. These catalysts can be homogeneous (in solution) or heterogeneous (solid phase), with heterogeneous catalysts being more common due to their ease of separation and recyclability.
- Types of Catalysts: Gasification catalysts can be classified based on their composition and mode of action. Common types include:
- Metal Catalysts: Metals such as nickel, cobalt, iron, ruthenium, and their oxides are widely used as catalysts for gasification reactions. They promote the water-gas shift reaction, where carbon monoxide reacts with steam to produce hydrogen and carbon dioxide, thus enhancing syngas hydrogen content.
- Alkali and Alkaline Earth Metals: Catalysts containing alkali (e.g., potassium, sodium) and alkaline earth metals (e.g., calcium, magnesium) are effective in enhancing gasification reactions by catalyzing tar cracking and inhibiting carbon deposition on catalyst surfaces.
- Mixed Metal Oxides: Catalysts composed of mixed metal oxides, such as cerium-zirconium, copper-zinc, and manganese-iron oxides, exhibit synergistic effects in promoting gasification reactions and improving syngas quality.
- Supported Catalysts: These catalysts are supported on porous materials such as alumina, silica, or zeolites, which provide a high surface area and enhance catalyst stability and activity.
- Catalyst Preparation: Gasification catalysts are prepared through various methods, including impregnation, precipitation, sol-gel synthesis, and co-precipitation. The choice of preparation method influences catalyst properties such as particle size, surface area, and dispersion, which in turn affect catalytic activity and selectivity.
- Catalyst Performance: The performance of gasification catalysts is evaluated based on several parameters, including catalytic activity, selectivity, stability, and resistance to deactivation. Catalyst deactivation mechanisms include fouling by tar and ash deposition, sintering (particle agglomeration), and poisoning by sulfur and trace contaminants present in the feedstock.
- Syngas Quality Enhancement: Gasification catalysts play a crucial role in enhancing syngas quality by promoting desirable reactions such as tar cracking, steam reforming, and water-gas shift. They also help mitigate the formation of undesirable by-products such as methane, ammonia, and volatile organic compounds (VOCs), thus improving syngas composition for downstream applications.
- Commercial Applications: Gasification catalysts find widespread applications in various industries, including power generation, chemical synthesis, and biofuel production. They are utilized in fixed-bed, fluidized-bed, and entrained-flow gasifiers for the production of syngas from coal, biomass, municipal solid waste, and other carbonaceous feedstocks.
In summary, gasification catalysts play a pivotal role in enhancing the efficiency, selectivity, and sustainability of gasification processes by promoting the conversion of solid carbonaceous materials into syngas. Continued research and development efforts are focused on developing novel catalyst formulations with improved activity, stability, and resistance to deactivation, thereby enabling the widespread adoption of gasification technologies for clean and efficient energy production.
Thermodynamics of Gasification:
The thermodynamics of gasification govern the energy transformations and equilibrium conditions during the conversion of solid carbonaceous materials into syngas. Understanding the thermodynamic principles underlying gasification processes is crucial for optimizing reactor design, operating conditions, and syngas composition. Here’s an in-depth exploration of the thermodynamics of gasification:
- Gibbs Free Energy: Gasification reactions proceed based on the principle of minimizing Gibbs free energy, which represents the maximum work that can be extracted from a system at constant temperature and pressure. For a gasification reaction to occur spontaneously, the change in Gibbs free energy (∆G) must be negative.
- Equilibrium Considerations: Gasification reactions are reversible and reach equilibrium under certain conditions. The equilibrium composition of syngas depends on factors such as temperature, pressure, feedstock composition, and catalyst presence. The equilibrium composition can be determined using thermodynamic equilibrium models such as the Gibbs free energy minimization approach.
- Reaction Kinetics: While thermodynamics govern the feasibility and equilibrium composition of gasification reactions, reaction kinetics dictate the rate at which these reactions occur. Kinetic factors such as activation energy, reaction mechanisms, and catalyst activity influence the overall gasification rate and the time required to reach equilibrium.
- Effect of Temperature: Gasification reactions are highly temperature-dependent, with higher temperatures favoring the decomposition of complex organic molecules and the formation of syngas constituents. However, excessive temperatures can lead to undesired side reactions, tar formation, and catalyst deactivation. Optimal gasification temperatures vary depending on the feedstock and gasification process employed.
- Pressure Effects: Gasification reactions are less sensitive to pressure variations compared to temperature changes. However, elevated pressures can enhance gasification rates by increasing reactant concentrations and promoting mass transfer. High-pressure gasification processes such as supercritical water gasification offer advantages in terms of syngas yield and purity.
- Feedstock Composition: The thermodynamics of gasification are influenced by the chemical composition and properties of the feedstock. Different feedstocks exhibit varying reactivity, tar content, and ash composition, leading to differences in gasification behavior and equilibrium composition.
- Syngas Composition: Thermodynamic calculations predict the equilibrium composition of syngas based on the feedstock composition and gasification conditions. Syngas composition is typically dominated by carbon monoxide (CO) and hydrogen (H2), with smaller amounts of carbon dioxide (CO2), methane (CH4), and other trace gases depending on the gasification process.
- Syngas Cleanup: Thermodynamic modeling can also inform the design of syngas cleanup systems to remove impurities such as tar, sulfur compounds, and particulate matter. Understanding the thermodynamics of gas-solid and gas-liquid equilibrium is crucial for designing effective cleanup processes.
- Energy Efficiency: By optimizing gasification conditions based on thermodynamic principles, energy efficiency and syngas quality can be maximized while minimizing environmental impacts. Advanced gasification technologies such as integrated gasification combined cycle (IGCC) systems leverage thermodynamic insights to achieve high overall energy conversion efficiencies.
In conclusion, the thermodynamics of gasification play a fundamental role in determining the feasibility, equilibrium composition, and efficiency of gasification processes. By applying thermodynamic principles, researchers and engineers can design and operate gasification systems that meet energy production goals while minimizing environmental footprints.
Catalytic Gasification:
Catalytic gasification involves the use of catalysts to facilitate the conversion of solid carbonaceous materials into syngas (a mixture of hydrogen and carbon monoxide) at lower temperatures and/or higher reaction rates compared to non-catalytic processes. Here’s a detailed exploration of catalytic gasification:
- Catalyst Types: Various types of catalysts can be employed in gasification processes, including metal catalysts (e.g., nickel, cobalt, iron), supported metal catalysts (e.g., nickel on alumina), and heterogeneous catalysts (e.g., zeolites, transition metal oxides). Catalyst selection depends on factors such as feedstock type, gasification temperature, desired syngas composition, and catalyst stability.
- Catalyst Function: Catalysts lower the activation energy required for gasification reactions, thereby enhancing reaction rates and promoting specific reaction pathways. They facilitate the decomposition of complex organic molecules present in the feedstock, promote tar cracking and reforming, and enhance the water-gas shift reaction for increased hydrogen production.
- Tar Reduction: One of the key advantages of catalytic gasification is its ability to reduce tar formation and improve syngas quality. Catalysts facilitate tar cracking and reforming reactions, breaking down tar compounds into smaller, more reactive species that can be further converted into syngas constituents. This helps mitigate operational issues such as equipment fouling and catalyst deactivation caused by tar deposition.
- Temperature Flexibility: Catalytic gasification can be carried out at lower temperatures compared to non-catalytic processes, allowing for better control over reaction kinetics and thermal management. Lower operating temperatures reduce energy consumption, minimize thermal stress on reactor materials, and enable the use of heat-sensitive feedstocks.
- Syngas Composition Control: Catalysts enable precise control over syngas composition by promoting selective gasification reactions and suppressing undesirable side reactions. For example, catalysts can enhance hydrogen production by favoring water-gas shift and steam reforming reactions while minimizing methane and tar formation.
- Catalyst Deactivation: Despite their benefits, catalysts are susceptible to deactivation over time due to factors such as coking, sintering, and poisoning by contaminants present in the feedstock or syngas. Strategies to mitigate catalyst deactivation include catalyst regeneration, periodic cleaning, catalyst formulation optimization, and the use of catalyst promoters and inhibitors.
- Integration with Other Processes: Catalytic gasification can be integrated with other downstream processes such as syngas cleanup, water-gas shift, and Fischer-Tropsch synthesis to produce a wide range of valuable products including hydrogen, ammonia, methanol, synthetic fuels, and chemicals. Catalyst selection and reactor design play critical roles in achieving desired product yields and selectivities.
- Environmental Benefits: Catalytic gasification offers environmental benefits such as reduced greenhouse gas emissions, improved air quality (due to lower emissions of particulate matter and volatile organic compounds), and efficient utilization of renewable and waste feedstocks. By converting biomass, coal, municipal solid waste, and other carbonaceous materials into clean syngas, catalytic gasification contributes to sustainable energy production and waste management.
In summary, catalytic gasification represents an advanced and versatile technology for converting solid carbonaceous materials into syngas with improved efficiency, flexibility, and environmental performance. Ongoing research and development efforts focus on further enhancing catalyst performance, understanding catalyst deactivation mechanisms, and optimizing process integration for commercial-scale applications.
Algal Biomass Gasification:
Algal biomass gasification involves the thermochemical conversion of algae-derived feedstock into syngas, a mixture primarily composed of hydrogen (H2) and carbon monoxide (CO). Here’s a detailed exploration of algal biomass gasification:
- Algal Biomass as Feedstock: Algae are diverse photosynthetic organisms that can grow rapidly in various aquatic environments using sunlight, water, and carbon dioxide (CO2). They possess high photosynthetic efficiency and can accumulate significant amounts of biomass, making them promising feedstock for bioenergy production.
- Gasification Process: Algal biomass gasification typically involves subjecting dried or pre-treated algae to elevated temperatures (>700°C) in the presence of a controlled amount of oxygen (O2) or steam (H2O) within a gasifier. The thermochemical reactions that occur during gasification include pyrolysis, oxidation, reduction, and gas-phase reforming.
- Feedstock Preparation: Prior to gasification, algal biomass may undergo various pre-treatment steps to enhance its suitability for conversion, such as drying to reduce moisture content, grinding to increase surface area, and torrefaction to improve energy density and chemical composition. These pre-treatment steps help optimize gasification performance and syngas quality.
- Gasification Reactions: During gasification, algal biomass undergoes a series of complex thermochemical reactions. Pyrolysis occurs initially, leading to the decomposition of organic matter into volatile compounds, char, and ash. Subsequent reactions involve the oxidation of char and volatile species, leading to the production of syngas constituents such as H2, CO, methane (CH4), and carbon dioxide (CO2).
- Syngas Composition: The composition of syngas produced from algal biomass gasification varies depending on factors such as feedstock composition, gasification conditions, and reactor design. Typically, syngas contains H2 and CO as primary constituents, with minor amounts of CH4, CO2, and trace impurities such as hydrogen sulfide (H2S) and ammonia (NH3).
- Syngas Utilization: The syngas produced from algal biomass gasification can be utilized in various downstream processes for energy generation and chemical production. Syngas can be combusted directly in gas turbines, reciprocating engines, or boilers to produce heat and power. Alternatively, it can undergo further processing via catalytic conversion or chemical synthesis to produce fuels and chemicals such as hydrogen, methanol, synthetic natural gas (SNG), and ammonia.
- Environmental Benefits: Algal biomass gasification offers several environmental benefits, including carbon neutrality, resource sustainability, and waste valorization. Algae can be cultivated using non-arable land and wastewater streams, reducing competition with food crops and freshwater resources. Additionally, algal biomass absorbs CO2 during growth, offsetting emissions generated during gasification and combustion processes.
- Challenges and Opportunities: Despite its potential, algal biomass gasification faces several challenges, including feedstock availability, cultivation scalability, energy density, and cost-effectiveness. Research efforts focus on optimizing cultivation techniques, improving biomass composition, enhancing gasification efficiency, and developing integrated biorefinery concepts to maximize value-added product yields.
In summary, algal biomass gasification represents a promising pathway for converting renewable biomass resources into syngas for clean energy and chemical production. Continued research and development efforts are essential to address technical, economic, and environmental challenges and unlock the full potential of algal biomass as a sustainable feedstock for gasification applications.
Catalytic Biomass Gasification:
Catalytic biomass gasification involves the use of catalysts to enhance the gasification process, resulting in improved reaction kinetics, higher gas yields, and enhanced syngas quality. Here’s an in-depth exploration of catalytic biomass gasification:
- Role of Catalysts: Catalysts play a crucial role in biomass gasification by facilitating chemical reactions, lowering activation energies, and promoting desired reaction pathways. They can accelerate the conversion of biomass into syngas while reducing tar formation, enhancing process efficiency, and enabling the utilization of lower temperatures and pressures.
- Types of Catalysts: Various types of catalysts are employed in biomass gasification, including transition metals (e.g., nickel, cobalt, iron), supported metal catalysts (e.g., supported on alumina, silica, or zeolites), mixed metal oxides, and biochar-based catalysts. Each type of catalyst exhibits specific catalytic properties and interactions with biomass-derived intermediates.
- Catalytic Mechanisms: Catalytic biomass gasification involves multiple catalytic mechanisms, including steam reforming, water-gas shift reaction, tar cracking, and char gasification. Steam reforming reactions involve the reaction of biomass-derived volatiles with steam to produce H2 and CO, while the water-gas shift reaction converts CO and steam into H2 and CO2. Tar cracking reactions break down complex organic compounds into smaller, more reactive species, reducing tar content in the syngas.
- Catalyst Preparation: Catalysts used in biomass gasification are typically prepared via impregnation, precipitation, co-precipitation, or deposition methods. The choice of preparation method influences catalyst properties such as surface area, particle size, dispersion, and active site density, which in turn affect catalytic performance and stability.
- Gasification Conditions: Catalytic biomass gasification can be conducted under a range of operating conditions, including temperature, pressure, steam-to-biomass ratio, and residence time. Optimal gasification conditions depend on catalyst type, feedstock composition, reactor configuration, and desired syngas specifications.
- Syngas Quality: The presence of catalysts in biomass gasification reactors can significantly influence syngas composition and quality. Catalytic biomass gasification typically yields syngas with higher H2 and CO content, reduced methane and tar concentrations, and improved stability compared to non-catalytic gasification processes.
- Catalyst Deactivation: Catalyst deactivation is a significant challenge in catalytic biomass gasification, resulting from factors such as coke deposition, sintering, poisoning, and chemical reactions with biomass constituents. Strategies to mitigate catalyst deactivation include catalyst regeneration, in-situ catalyst activation, and the use of novel catalyst materials with improved stability and resistance to deactivation.
- Applications: Catalytic biomass gasification has diverse applications in bioenergy production, renewable hydrogen generation, syngas upgrading, and chemical synthesis. It enables the efficient utilization of biomass resources for power generation, heat production, transportation fuels, and value-added chemicals, contributing to the transition towards a sustainable and low-carbon economy.
In summary, catalytic biomass gasification offers a promising approach to convert biomass into syngas with improved efficiency, syngas quality, and process flexibility. Continued research and development efforts are essential to advance catalyst design, optimize gasification processes, and overcome challenges associated with catalyst deactivation, paving the way for the widespread adoption of catalytic biomass gasification technologies.
Hydrothermal Carbonization (HTC):
Hydrothermal carbonization (HTC) is a thermochemical process that converts wet biomass into a carbon-rich solid called hydrochar under high temperature and pressure conditions in the presence of water. Here’s an in-depth exploration of hydrothermal carbonization:
- Principle: Hydrothermal carbonization mimics the natural process of coal formation by subjecting biomass to elevated temperatures (180-250°C) and pressures (5-40 bar) in an aqueous environment. The process transforms biomass polymers into hydrochar through dehydration, decarboxylation, condensation, and carbonization reactions, resulting in the formation of a solid carbonaceous material.
- Feedstock Compatibility: HTC can utilize a wide range of wet biomass feedstocks, including sewage sludge, agricultural residues, organic waste, algae, and lignocellulosic biomass. Unlike conventional thermochemical processes, HTC does not require extensive drying of feedstock, making it suitable for processing high-moisture biomass streams and organic waste materials.
- Reaction Pathways: During hydrothermal carbonization, biomass undergoes a series of complex chemical reactions driven by temperature, pressure, and reaction time. These reactions include hydrolysis, dehydration, decarboxylation, polymerization, and aromatization, leading to the conversion of biomass constituents into carbon-rich solids, liquid by-products (aqueous phase), and gaseous compounds (off-gas).
- Process Parameters: Key process parameters in hydrothermal carbonization include temperature, pressure, residence time, biomass-to-water ratio, and pH. Optimal process conditions vary depending on feedstock characteristics, desired hydrochar properties, and specific application requirements. Higher temperatures and longer reaction times generally result in increased carbonization and hydrochar yield.
- Hydrochar Properties: The properties of hydrochar produced via HTC are influenced by feedstock composition, process conditions, and post-treatment methods. Hydrochar typically exhibits high carbon content (>50%), low moisture content (<10%), high energy density, and improved stability compared to raw biomass. Its physicochemical properties can be tailored for various applications, including soil amendment, carbon sequestration, energy production, and wastewater treatment.
- Applications: Hydrothermal carbonization has diverse applications in agriculture, environmental remediation, renewable energy, and resource recovery. Hydrochar can be used as a soil conditioner to improve soil fertility, water retention, and carbon sequestration in agricultural and forestry systems. It can also serve as a renewable energy source through combustion, gasification, or pyrolysis processes.
- Environmental Benefits: HTC offers several environmental benefits, including the diversion of organic waste from landfills, reduction of greenhouse gas emissions, and mitigation of nutrient runoff and soil erosion. By converting biomass into stable carbonaceous materials, hydrothermal carbonization contributes to the circular economy and promotes sustainable waste management practices.
- Challenges and Opportunities: Despite its potential, hydrothermal carbonization faces challenges related to process optimization, scale-up, feedstock logistics, and product utilization. Continued research efforts are needed to enhance process efficiency, develop cost-effective technologies, and explore novel applications for hydrochar-derived products in various sectors.
In summary, hydrothermal carbonization is a promising thermochemical conversion technology for valorizing wet biomass and organic waste streams into valuable hydrochar products. By integrating biomass processing with water-based chemistry, HTC offers a sustainable pathway towards resource recovery, carbon sequestration, and renewable energy production, contributing to the transition to a circular and low-carbon economy.
Carbonization:
Carbonization is a thermochemical process that converts organic materials into carbon-rich solids, gases, and liquids by subjecting them to high temperatures in the absence of oxygen. This process results in the decomposition and transformation of biomass into char, bio-oil, and syngas, offering various applications in energy production, materials synthesis, and environmental remediation. Here’s an in-depth look at carbonization:
- Principle: Carbonization involves heating biomass in a controlled environment with limited or no oxygen supply to prevent combustion. The absence of oxygen inhibits complete oxidation, leading to the breakdown of organic compounds into carbonaceous residues and volatile by-products. The process typically occurs at temperatures ranging from 300°C to 900°C, depending on the feedstock and desired products.
- Feedstock Diversity: Carbonization can utilize a wide range of feedstocks, including wood, agricultural residues, municipal solid waste, sewage sludge, and biomass pellets. Each feedstock has unique chemical compositions and thermal properties, influencing the carbonization process’s efficiency and the quality of the resulting products.
- Process Variants: Carbonization encompasses various techniques, including slow pyrolysis, fast pyrolysis, torrefaction, and hydrothermal carbonization, each offering distinct advantages and limitations. Slow pyrolysis involves gradual heating of biomass at low temperatures (300-500°C) over an extended period, resulting in higher char yields and bio-oil production. In contrast, fast pyrolysis operates at higher temperatures (500-900°C) and shorter residence times, favoring the production of bio-oil with minimal char formation.
- Product Formation: During carbonization, biomass undergoes thermal decomposition and chemical reactions, leading to the formation of three primary products: biochar (solid carbonaceous residue), bio-oil (liquid fraction), and syngas (gaseous mixture). The composition and yield of these products depend on factors such as feedstock properties, process conditions, and reactor design.
- Biochar Properties: Biochar produced via carbonization exhibits diverse physicochemical properties, including high carbon content (>50%), porosity, surface area, and stability. These properties make biochar suitable for various applications, such as soil amendment, carbon sequestration, water filtration, and energy storage. Biochar’s porous structure enhances soil fertility, moisture retention, and nutrient cycling, promoting sustainable agriculture and mitigating climate change.
- Bio-oil Characteristics: Bio-oil obtained from carbonization contains a complex mixture of organic compounds, including phenols, aldehydes, ketones, and aromatic hydrocarbons. It has potential applications as a renewable fuel, chemical feedstock, or biorefinery intermediate. However, bio-oil’s high oxygen content, acidity, and instability pose challenges for storage, transportation, and utilization, requiring further upgrading or refining processes.
- Syngas Utilization: Syngas produced during carbonization consists mainly of carbon monoxide (CO) and hydrogen (H2), along with trace amounts of methane (CH4) and other gases. Syngas can be utilized for heat and power generation through combustion, gasification, or syngas fermentation processes. It serves as a versatile feedstock for synthesizing fuels, chemicals, and value-added products in the chemical, petrochemical, and bioenergy industries.
- Environmental Impact: Carbonization offers several environmental benefits, including carbon sequestration, waste valorization, and renewable energy production. By converting biomass into stable carbonaceous materials (e.g., biochar), carbonization contributes to carbon dioxide (CO2) mitigation and soil improvement, thereby enhancing ecosystem resilience and mitigating climate change impacts.
- Challenges and Opportunities: Despite its potential, carbonization faces challenges related to feedstock availability, process optimization, product quality, and economic viability. Research efforts are needed to improve process efficiency, develop advanced reactor technologies, and explore novel applications for carbonaceous materials in emerging sectors such as energy storage, carbon capture, and sustainable manufacturing.
In summary, carbonization is a versatile thermochemical conversion process for converting biomass into valuable products, including biochar, bio-oil, and syngas. With advancements in technology and research, carbonization holds promise for addressing global challenges related to waste management, renewable energy, and environmental sustainability, paving the way towards a circular and low-carbon economy.
Gasification:
Gasification is a thermochemical process that converts carbonaceous materials, such as biomass, coal, or organic waste, into a synthesis gas (syngas) comprising hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and trace amounts of other gases. This process occurs under controlled conditions of temperature, pressure, and gas composition, typically in the presence of a gasification agent (e.g., air, oxygen, steam) to facilitate the chemical reactions. Here’s an in-depth exploration of gasification:
- Principle: Gasification involves the partial oxidation of carbonaceous feedstocks at elevated temperatures (>700°C) in a controlled environment with limited oxygen supply. The primary reactions include combustion, pyrolysis, and reforming, leading to the conversion of solid carbonaceous materials into a gaseous mixture of combustible gases and by-products.
- Feedstock Diversity: Gasification can utilize a wide range of feedstocks, including biomass (e.g., wood chips, agricultural residues), coal, municipal solid waste, and industrial waste streams. The choice of feedstock influences the gasification process’s performance, product composition, and overall energy efficiency.
- Process Variants: Gasification technologies encompass various process configurations, such as fixed-bed, fluidized-bed, entrained-flow, and plasma gasification systems. Each configuration offers specific advantages in terms of feedstock flexibility, gasification efficiency, tar removal, and syngas quality, depending on the application requirements and feedstock characteristics.
- Gasification Reactions: Gasification involves several complex thermochemical reactions, including pyrolysis (thermal decomposition of biomass), oxidation (combustion of char and volatiles), steam reforming (water-gas reaction), and shift reactions (conversion of CO and water vapor to CO2 and H2). These reactions occur sequentially, producing syngas with varying compositions depending on the operating conditions and feedstock properties.
- Syngas Composition: The composition of syngas generated from gasification depends on factors such as feedstock type, gasification agent, temperature, and residence time. Typically, syngas contains hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and trace amounts of hydrogen sulfide (H2S), ammonia (NH3), and tars. The syngas composition can be adjusted by controlling process parameters to meet specific downstream applications, such as fuel synthesis or power generation.
- Syngas Utilization: Syngas produced from gasification has diverse applications in heat and power generation, fuel synthesis (e.g., Fischer-Tropsch synthesis), chemical production (e.g., methanol, ammonia), and hydrogen production. It serves as a versatile feedstock for producing renewable fuels, chemicals, and value-added products, offering opportunities for energy diversification, carbon mitigation, and waste valorization.
- Tar Formation and Removal: Tar compounds, formed during the gasification process, can cause equipment fouling, corrosion, and syngas quality degradation. Tar removal techniques include physical methods (e.g., filtration, condensation), chemical methods (e.g., catalytic cracking, scrubbing), and thermal methods (e.g., tar cracking). Effective tar removal is essential for ensuring the long-term operability and efficiency of gasification systems.
- Environmental Impact: Gasification offers several environmental benefits, including reduced greenhouse gas emissions, waste diversion from landfills, and resource conservation. By converting biomass and waste materials into syngas, gasification contributes to energy recovery, renewable energy integration, and sustainable waste management practices, thereby mitigating environmental pollution and resource depletion.
- Challenges and Opportunities: Despite its potential, gasification faces challenges related to technology maturity, feedstock variability, process complexity, and economic viability. Research efforts are needed to address these challenges and advance gasification technology towards commercial deployment, optimization, and integration with other renewable energy systems.
In summary, gasification is a versatile thermochemical conversion process for converting carbonaceous feedstocks into syngas, offering opportunities for energy production, waste valorization, and sustainable development. With ongoing advancements in gasification technology and research, the potential of gasification to contribute to global energy security, environmental sustainability, and economic growth continues to grow.
Sustainable Energy:
Sustainable energy refers to energy sources and systems that meet present energy needs without compromising the ability of future generations to meet their own needs. It encompasses renewable energy sources, energy efficiency measures, and responsible energy management practices aimed at minimizing environmental impacts, conserving natural resources, and fostering social and economic development. Here’s a detailed exploration of sustainable energy:
- Renewable Energy Sources: Sustainable energy relies predominantly on renewable energy sources, including solar, wind, hydroelectric, biomass, geothermal, and tidal energy. These sources harness natural processes or phenomena to generate electricity, heat, or mechanical power, offering abundant, clean, and inexhaustible energy options compared to finite fossil fuels.
- Environmental Benefits: Renewable energy sources produce minimal greenhouse gas emissions and pollutants during energy generation, contributing to mitigating climate change, reducing air pollution, and preserving ecosystems. They help mitigate environmental degradation, biodiversity loss, and ecosystem disruption associated with fossil fuel extraction, combustion, and waste disposal.
- Energy Efficiency: Sustainable energy emphasizes energy efficiency measures and technologies aimed at optimizing energy use, minimizing energy losses, and improving energy productivity across various sectors, including buildings, transportation, industry, and agriculture. Energy efficiency measures reduce energy consumption, operating costs, and environmental impacts, while enhancing energy security and resilience.
- Decentralized Energy Systems: Sustainable energy promotes decentralized energy systems characterized by distributed generation, microgrids, and local energy resources, enabling communities, businesses, and households to generate, store, and manage their energy supply autonomously. Decentralized energy systems enhance energy access, reliability, and resilience, particularly in remote or underserved areas.
- Technological Innovations: Advances in renewable energy technologies, energy storage systems, smart grids, and digitalization drive the transition towards sustainable energy systems. Technological innovations enhance the performance, reliability, and affordability of renewable energy systems, enabling their integration into existing energy infrastructure and grid networks.
- Policy Support: Sustainable energy policies and regulations play a crucial role in promoting renewable energy deployment, incentivizing energy efficiency investments, and creating favorable market conditions for sustainable energy adoption. Policy measures include feed-in tariffs, renewable energy targets, carbon pricing mechanisms, energy efficiency standards, and financial incentives.
- Economic Opportunities: The transition to sustainable energy presents significant economic opportunities, including job creation, economic diversification, and investment attraction in renewable energy industries, supply chains, and related sectors. Renewable energy deployment stimulates innovation, entrepreneurship, and economic growth, contributing to energy security and competitiveness.
- Social Equity: Sustainable energy initiatives prioritize social equity, ensuring equitable access to clean, affordable, and reliable energy services for all communities, including marginalized and underserved populations. Energy access initiatives, rural electrification programs, and community-owned renewable energy projects promote social inclusion, poverty reduction, and human development.
- Global Energy Transition: The global energy transition towards sustainable energy is driven by international cooperation, multilateral agreements, and collective efforts to address climate change, energy poverty, and environmental degradation. Initiatives such as the Paris Agreement, Sustainable Development Goals (SDGs), and Clean Energy Ministerial (CEM) facilitate cooperation among governments, businesses, and civil society to accelerate the adoption of sustainable energy solutions.
In summary, sustainable energy represents a holistic approach to meeting current and future energy needs in a manner that fosters environmental stewardship, social equity, and economic prosperity. By embracing renewable energy sources, energy efficiency measures, and responsible energy management practices, societies can achieve sustainable development goals, combat climate change, and create a more resilient and equitable energy future for all.
Tar Removal:
Tar removal is a critical process in various industries, particularly those involving the utilization of biomass or fossil fuels. Tar, a complex mixture of organic compounds, is often formed during the thermal conversion of organic materials, such as biomass gasification, pyrolysis, or coal gasification. It can also be encountered in industrial processes like oil refining, gasification, and combustion. Here’s an in-depth exploration of tar removal:
- Formation of Tar: Tar is formed as a byproduct of incomplete combustion or thermal decomposition of organic materials. During high-temperature processes like gasification or pyrolysis, volatile organic compounds released from biomass or coal undergo complex chemical reactions, leading to the formation of tar compounds. Tar consists of various hydrocarbons, oxygenates, and other organic compounds, including phenols, polycyclic aromatic hydrocarbons (PAHs), and oxygen-containing compounds like aldehydes and ketones.
- Impacts of Tar: Tar poses several challenges in industrial processes and applications. It can condense and deposit on equipment surfaces, causing fouling, corrosion, and erosion in gasifiers, boilers, engines, and downstream processing units. Tar-containing gases may also lead to tar-related issues in gas cleanup systems, catalyst deactivation, and environmental emissions. Therefore, efficient tar removal is essential to ensure the reliable and sustainable operation of energy conversion processes.
- Tar Removal Technologies: Various technologies are available for tar removal, depending on the specific process conditions, tar composition, and desired product quality. Common tar removal methods include physical separation, chemical scrubbing, catalytic conversion, thermal cracking, and filtration. Each method has its advantages, limitations, and applicability to different process configurations and feedstock types.
- Physical Separation: Physical separation techniques involve the cooling and condensation of tar-containing gases to recover tar as a liquid or solid product. Condensation methods utilize low temperatures to liquefy tar compounds, which can then be separated by gravity settling, centrifugation, or filtration. Cyclones, scrubbers, and electrostatic precipitators are also employed to remove tar particulates from gas streams.
- Chemical Scrubbing: Chemical scrubbing processes utilize solvents or reactive agents to chemically absorb or react with tar compounds, converting them into soluble or less harmful species. Water scrubbing, alkali scrubbing, and acid scrubbing are common chemical scrubbing methods used for tar removal. These processes are effective for removing water-soluble tars, phenolic compounds, and acidic constituents from gas streams.
- Catalytic Conversion: Catalytic tar reforming involves the use of catalysts to promote the cracking, reforming, or hydrogenation of tar compounds into lighter hydrocarbons or gases. Catalytic tar removal processes operate at elevated temperatures and pressures, typically in the presence of steam or hydrogen gas. Catalysts such as nickel, cobalt, or transition metal oxides are used to enhance tar conversion rates and selectivity.
- Thermal Cracking: Thermal cracking or pyrolysis of tar involves subjecting tar-containing gases to high temperatures and residence times to thermally decompose tar molecules into smaller, less complex compounds. Tar cracking processes may occur spontaneously at high temperatures or be facilitated by catalysts or thermal cracking reactors. Thermal cracking can significantly reduce tar concentrations in gas streams, but it requires careful control of process parameters to avoid equipment fouling and coke formation.
- Filtration: Filtration methods utilize porous materials or membranes to physically capture and remove tar particulates from gas streams. Filtration media such as ceramic filters, metallic filters, or fibrous materials are employed to trap tar particles while allowing clean gases to pass through. Filtration is often used as a final polishing step in gas cleanup systems to achieve stringent tar removal efficiency requirements.
- Integration with Gasification Processes: Tar removal technologies are integrated into gasification systems to ensure the production of clean syngas suitable for downstream applications like power generation, chemical synthesis, or biofuel production. Gas cleanup systems typically comprise multiple tar removal stages, including primary gas cooling, scrubbing, catalytic conversion, and particulate filtration, to achieve the desired syngas quality and purity.
- Challenges and Considerations: Despite the advancements in tar removal technologies, challenges such as catalyst deactivation, corrosion, fouling, and energy consumption remain significant concerns in tar removal processes. Additionally, the composition and properties of tar compounds vary widely depending on feedstock composition, process conditions, and operating parameters, requiring tailored tar removal solutions for different applications.
In summary, tar removal is a critical step in various industrial processes involving biomass or fossil fuel conversion. Effective tar removal technologies are essential for ensuring the reliable operation, environmental compliance, and product quality in gasification, pyrolysis, and other energy conversion processes. Continued research and innovation in tar removal technologies are necessary to address existing challenges and enhance the efficiency and sustainability of energy conversion systems.
Tar Removal:
Tar removal is a crucial process in various industries, particularly those involving the utilization of biomass or fossil fuels. Tar, a complex mixture of organic compounds, is often formed during the thermal conversion of organic materials, such as biomass gasification, pyrolysis, or coal gasification. It can also be encountered in industrial processes like oil refining, gasification, and combustion. Here’s an in-depth exploration of tar removal:
- Formation of Tar: Tar is formed as a byproduct of incomplete combustion or thermal decomposition of organic materials. During high-temperature processes like gasification or pyrolysis, volatile organic compounds released from biomass or coal undergo complex chemical reactions, leading to the formation of tar compounds. Tar consists of various hydrocarbons, oxygenates, and other organic compounds, including phenols, polycyclic aromatic hydrocarbons (PAHs), and oxygen-containing compounds like aldehydes and ketones.
- Impacts of Tar: Tar poses several challenges in industrial processes and applications. It can condense and deposit on equipment surfaces, causing fouling, corrosion, and erosion in gasifiers, boilers, engines, and downstream processing units. Tar-containing gases may also lead to tar-related issues in gas cleanup systems, catalyst deactivation, and environmental emissions. Therefore, efficient tar removal is essential to ensure the reliable and sustainable operation of energy conversion processes.
- Tar Removal Technologies: Various technologies are available for tar removal, depending on the specific process conditions, tar composition, and desired product quality. Common tar removal methods include physical separation, chemical scrubbing, catalytic conversion, thermal cracking, and filtration. Each method has its advantages, limitations, and applicability to different process configurations and feedstock types.
- Physical Separation: Physical separation techniques involve the cooling and condensation of tar-containing gases to recover tar as a liquid or solid product. Condensation methods utilize low temperatures to liquefy tar compounds, which can then be separated by gravity settling, centrifugation, or filtration. Cyclones, scrubbers, and electrostatic precipitators are also employed to remove tar particulates from gas streams.
- Chemical Scrubbing: Chemical scrubbing processes utilize solvents or reactive agents to chemically absorb or react with tar compounds, converting them into soluble or less harmful species. Water scrubbing, alkali scrubbing, and acid scrubbing are common chemical scrubbing methods used for tar removal. These processes are effective for removing water-soluble tars, phenolic compounds, and acidic constituents from gas streams.
- Catalytic Conversion: Catalytic tar reforming involves the use of catalysts to promote the cracking, reforming, or hydrogenation of tar compounds into lighter hydrocarbons or gases. Catalytic tar removal processes operate at elevated temperatures and pressures, typically in the presence of steam or hydrogen gas. Catalysts such as nickel, cobalt, or transition metal oxides are used to enhance tar conversion rates and selectivity.
- Thermal Cracking: Thermal cracking or pyrolysis of tar involves subjecting tar-containing gases to high temperatures and residence times to thermally decompose tar molecules into smaller, less complex compounds. Tar cracking processes may occur spontaneously at high temperatures or be facilitated by catalysts or thermal cracking reactors. Thermal cracking can significantly reduce tar concentrations in gas streams, but it requires careful control of process parameters to avoid equipment fouling and coke formation.
- Filtration: Filtration methods utilize porous materials or membranes to physically capture and remove tar particulates from gas streams. Filtration media such as ceramic filters, metallic filters, or fibrous materials are employed to trap tar particles while allowing clean gases to pass through. Filtration is often used as a final polishing step in gas cleanup systems to achieve stringent tar removal efficiency requirements.
- Integration with Gasification Processes: Tar removal technologies are integrated into gasification systems to ensure the production of clean syngas suitable for downstream applications like power generation, chemical synthesis, or biofuel production. Gas cleanup systems typically comprise multiple tar removal stages, including primary gas cooling, scrubbing, catalytic conversion, and particulate filtration, to achieve the desired syngas quality and purity.
- Challenges and Considerations: Despite the advancements in tar removal technologies, challenges such as catalyst deactivation, corrosion, fouling, and energy consumption remain significant concerns in tar removal processes. Additionally, the composition and properties of tar compounds vary widely depending on feedstock composition, process conditions, and operating parameters, requiring tailored tar removal solutions for different applications.
In summary, tar removal is a critical step in various industrial processes involving biomass or fossil fuel conversion. Effective tar removal technologies are essential for ensuring the reliable operation, environmental compliance, and product quality in gasification, pyrolysis, and other energy conversion processes. Continued research and innovation in tar removal technologies are necessary to address existing challenges and enhance the efficiency and sustainability of energy conversion systems.
EMS Power Machines
We design, manufacture and assembly Power Machines such as – diesel generators, electric motors, vibration motors, pumps, steam engines and steam turbines
EMS Power Machines is a global power engineering company, one of the five world leaders in the industry in terms of installed equipment. The companies included in the company have been operating in the energy market for more than 60 years.
EMS Power Machines manufactures steam turbines, gas turbines, hydroelectric turbines, generators, and other power equipment for thermal, nuclear, and hydroelectric power plants, as well as for various industries, transport, and marine energy.
EMS Power Machines is a major player in the global power industry, and its equipment is used in power plants all over the world. The company has a strong track record of innovation, and it is constantly developing new and improved technologies.
Here are some examples of Power Machines’ products and services:
- Steam turbines for thermal and nuclear power plants
- Gas turbines for combined cycle power plants and industrial applications
- Hydroelectric turbines for hydroelectric power plants
- Generators for all types of power plants
- Boilers for thermal power plants
- Condensers for thermal power plants
- Reheaters for thermal power plants
- Air preheaters for thermal power plants
- Feedwater pumps for thermal power plants
- Control systems for power plants
- Maintenance and repair services for power plants
EMS Power Machines is committed to providing its customers with high-quality products and services. The company has a strong reputation for reliability and innovation. Power Machines is a leading provider of power equipment and services, and it plays a vital role in the global power industry.
EMS Power Machines, which began in 1961 as a small factory of electric motors, has become a leading global supplier of electronic products for different segments. The search for excellence has resulted in the diversification of the business, adding to the electric motors products which provide from power generation to more efficient means of use.
